Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -
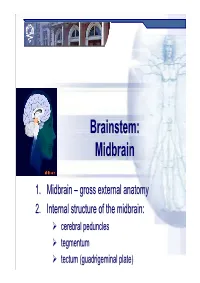
Brainstem: Midbrainmidbrain
Brainstem:Brainstem: MidbrainMidbrain 1.1. MidbrainMidbrain –– grossgross externalexternal anatomyanatomy 2.2. InternalInternal structurestructure ofof thethe midbrain:midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain MidbrainMidbrain –– generalgeneral featuresfeatures location – between forebrain and hindbrain the smallest region of the brainstem – 6-7g the shortest brainstem segment ~ 2 cm long least differentiated brainstem division human midbrain is archipallian – shared general architecture with the most ancient of vertebrates embryonic origin – mesencephalon main functions:functions a sort of relay station for sound and visual information serves as a nerve pathway of the cerebral hemispheres controls the eye movement involved in control of body movement Prof. Dr. Nikolai Lazarov 2 Midbrain MidbrainMidbrain –– grossgross anatomyanatomy dorsal part – tectum (quadrigeminal plate): superior colliculi inferior colliculi cerebral aqueduct ventral part – cerebral peduncles:peduncles dorsal – tegmentum (central part) ventral – cerebral crus substantia nigra Prof. Dr. Nikolai Lazarov 3 Midbrain CerebralCerebral cruscrus –– internalinternal structurestructure CerebralCerebral peduncle:peduncle: crus cerebri tegmentum mesencephali substantia nigra two thick semilunar white matter bundles composition – somatotopically arranged motor tracts: corticospinal } pyramidal tracts – medial ⅔ corticobulbar corticopontine fibers: frontopontine tracts – medially temporopontine tracts – laterally -

Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -
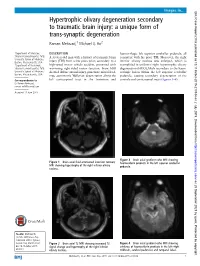
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

Closed-Loop Neuromodulation Restores Network Connectivity And
RESEARCH ARTICLE Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury Patrick D Ganzer1,2*, Michael J Darrow1, Eric C Meyers1,2, Bleyda R Solorzano2, Andrea D Ruiz2, Nicole M Robertson2, Katherine S Adcock3, Justin T James2, Han S Jeong2, April M Becker4, Mark P Goldberg4, David T Pruitt1,2, Seth A Hays1,2,3, Michael P Kilgard1,2,3, Robert L Rennaker II1,2,3* 1Erik Jonsson School of Engineering and Computer Science, The University of Texas at Dallas, Richardson, United States; 2Texas Biomedical Device Center, Richardson, United States; 3School of Behavioral Brain Sciences, The University of Texas at Dallas, Richardson, United States; 4Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, United States Abstract Recovery from serious neurological injury requires substantial rewiring of neural circuits. Precisely-timed electrical stimulation could be used to restore corrective feedback mechanisms and promote adaptive plasticity after neurological insult, such as spinal cord injury (SCI) or stroke. This study provides the first evidence that closed-loop vagus nerve stimulation (CLV) based on the synaptic eligibility trace leads to dramatic recovery from the most common forms of SCI. The addition of CLV to rehabilitation promoted substantially more recovery of forelimb function compared to rehabilitation alone following chronic unilateral or bilateral cervical SCI in a rat model. Triggering stimulation on the most successful movements is critical to maximize recovery. CLV enhances recovery by strengthening synaptic connectivity from remaining motor *For correspondence: networks to the grasping muscles in the forelimb. The benefits of CLV persist long after the end of [email protected] stimulation because connectivity in critical neural circuits has been restored. -

Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: a Study Using a High-Resolution Semiconductor PET System
Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: A Study Using a High-Resolution Semiconductor PET System Kenji Hirata1,2, Naoya Hattori3, Wataru Takeuchi4, Tohru Shiga1, Yuichi Morimoto4, Kikuo Umegaki5, Kentaro Kobayashi1, Osamu Manabe1, Shozo Okamoto1, and Nagara Tamaki1 1Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA, Los Angeles, California; 3Department of Molecular Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 4Research and Development Group, Hitachi Ltd., Tokyo, Japan; and 5Division of Quantum Science and Engineering, Faculty of Engineering, Hokkaido University, Sapporo, Japan (1). The rubrospinal tract mainly controls limb musculature, whereas The red nucleus (RN) is a pair of small gray matter structures located the pyramidal tract can act on the whole musculature (1). A recent in the midbrain and involved in muscle movement and cognitive anatomic study indicated that a significant amount of rubrospinal tract functions. This retrospective study aimed to investigate the metabo- is present in the human brain (2). Because neuronal activity of the RN lism of human RN and its correlation to other brain regions. Methods: in Parkinson disease is known to be increased during passive and We developed a high-resolution semiconductor PET system to image voluntary movements (3), the RN may play a role in the coordination small brain structures. Twenty patients without neurologic disorders of muscular movement. Cognitive symptoms, such as intellectual underwent whole-brain scanning after injection of 400 MBq of fatigability, decreased verbal fluency, and discrete memory impair- 18F-FDG. -

Functional Neuroanatomy for Posture and Gait Control
REVIEW ARTICLE https://doi.org/10.14802/jmd.16062 / J Mov Disord 2017;10(1):1-17 pISSN 2005-940X / eISSN 2093-4939 Functional ABSTRACT Here we argue functional neuroanatomy for pos- ture-gait control. Multi-sensory information such as Neuroanatomy somatosensory, visual and vestibular sensation act on various areas of the brain so that adaptable pos- ture-gait control can be achieved. Automatic process for Posture and of gait, which is steady-state stepping movements associating with postural reflexes including head- eye coordination accompanied by appropriate align- Gait Control ment of body segments and optimal level of pos- tural muscle tone, is mediated by the descending pathways from the brainstem to the spinal cord. Par- ticularly, reticulospinal pathways arising from the lat- Kaoru Takakusaki eral part of the mesopontine tegmentum and spinal locomotor network contribute to this process. On The Research Center for Brain Function and Medical Engineering, the other hand, walking in unfamiliar circumstance Asahikawa Medical University, Asahikawa, Japan requires cognitive process of postural control, which depends on knowledges of self-body, such as body schema and body motion in space. The cognitive in- formation is produced at the temporoparietal asso- ciation cortex, and is fundamental to sustention of vertical posture and construction of motor programs. The programs in the motor cortical areas run to ex- ecute anticipatory postural adjustment that is opti- mal for achievement of goal-directed movements. The basal ganglia and cerebellum may affect both the automatic and cognitive processes of posture- gait control through reciprocal connections with the brainstem and cerebral cortex, respectively. Conse- quently, impairments in cognitive function by dam- ages in the cerebral cortex, basal ganglia and cere- bellum may disturb posture-gait control, resulting in falling. -

Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study
Original Article J. Phys. Ther. Sci. 22: 7–10, 2010 Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study SUNG HO JANG, MD1), JI HEON HONG, PT, MS1), YONG HYUN KWON, PT, PhD2) 1)Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 2)Department of Physical Therapy, Yeungnam College of Science & Technology: 1737, Daemyung 7-Dong, Namgu, Daegu, 705-703, Republic of Korea. TEL +82 53-650-9702, FAX: +82 53-629-5048 Abstract. [Purpose] Little is known about the rubro-olivary tract (ROT) in the human brain. We attempted to identify the ROT using diffusion tensor tractography (DTT). [Subjects and Methods] We obtained ROT data from 11 healthy subjects with no history of neurological disorder. For tracking of the ROT, a seed region of interest (ROI) was selected in the red nucleus, and a target ROI was found in the inferior olive of each subject. [Results] The ROT, originated in the red nucleus, and passed laterally to the decussation of the superior cerebellar peduncle in the lower midbrain. In the pons, it descended through the area adjacent to the medial lemnicus in the posterior direction. Within the medulla, the ROT ended in the inferior olive, which was located lateral to the medial lemnicus and posterior to the pyramid. [Conclusion] We identified the ROT in the human brain using DTT. These results will be informative for research into the ROT in the human brain. Key words: Diffusion tensor image, Red nucleus, Inferior olive (This article was submitted Jul. 24, 2009, and was accepted Sep. -

Basal Ganglia and Cerebellum Receive Different Somatosensory Information in Rats (Barrel Field/Pontine Nuclei/Cortical Lamnation/Vibrissae) BARBARA E
Proc. Nati. Acad. Sci. USA Vol. 87, pp. 4388-4392, June 1990 Neurobiology Basal ganglia and cerebellum receive different somatosensory information in rats (barrel field/pontine nuclei/cortical lamnation/vibrissae) BARBARA E. MERCIER*, CHARLES R. LEGGt, AND MITCHELL GLICKSTEIN Department of Anatomy and Developmental Biology, University College London, Gower Street, London WC1, United Kingdom Communicated by Irving T. Diamond, March 14, 1990 ABSTRACT There are two great subcortical circuits that sublamina Vb stain far more densely, suggesting that they are relay sensory information to motor structures in the mamma- capable ofhigher levels oftonic activity than those in Va (12). lian brain. One pathway relays via the pontine nuclei and Previous study of the efferent pathways from the rat cerebellum, and the other relays by way of the basal ganglia. somatosensory cortex (2) has suggested that the cell popu- We studied the cells oforigin ofthese two major pathways from lation projecting to the basal ganglia is centered on lamina Va, the posteromedial barrel subfield ofrats, a distinct region ofthe while the population projecting to the cerebellum is centered somatosensory cortex that contains the sensory representation on Vb. However, the methods available at the time the study of the large whiskers. We itjected tracer substances into the was done were relatively insensitive (13) and a comparison caudate putamen or the pontine nuclei and charted the location with more recent work (14) shows that only a subset of the of retrogradely filled cortical cells. In preliminary studies, we entire population of corticopontine cells was identified. Wise used double-labeling techniques to determine whether the cells and Jones (figure 6a in ref. -

Chapter 3: Internal Anatomy of the Central Nervous System
10353-03_CH03.qxd 8/30/07 1:12 PM Page 82 3 Internal Anatomy of the Central Nervous System LEARNING OBJECTIVES Nuclear structures and fiber tracts related to various functional systems exist side by side at each level of the After studying this chapter, students should be able to: nervous system. Because disease processes in the brain • Identify the shapes of corticospinal fibers at different rarely strike only one anatomic structure or pathway, there neuraxial levels is a tendency for a series of related and unrelated clinical symptoms to emerge after a brain injury. A thorough knowl- • Recognize the ventricular cavity at various neuroaxial edge of the internal brain structures, including their shape, levels size, location, and proximity, makes it easier to understand • Recognize major internal anatomic structures of the their functional significance. In addition, the proximity of spinal cord and describe their functions nuclear structures and fiber tracts explains multiple symp- toms that may develop from a single lesion site. • Recognize important internal anatomic structures of the medulla and explain their functions • Recognize important internal anatomic structures of the ANATOMIC ORIENTATION pons and describe their functions LANDMARKS • Identify important internal anatomic structures of the midbrain and discuss their functions Two distinct anatomic landmarks used for visual orientation to the internal anatomy of the brain are the shapes of the • Recognize important internal anatomic structures of the descending corticospinal fibers and the ventricular cavity forebrain (diencephalon, basal ganglia, and limbic (Fig. 3-1). Both are present throughout the brain, although structures) and describe their functions their shape and size vary as one progresses caudally from the • Follow the continuation of major anatomic structures rostral forebrain (telencephalon) to the caudal brainstem. -

The Long Journey of Pontine Nuclei Neurons : from Rhombic Lip To
REVIEW Erschienen in: Frontiers in Neural Circuits ; 11 (2017). - 33 published: 17 May 2017 http://dx.doi.org/10.3389/fncir.2017.00033 doi: 10.3389/fncir.2017.00033 The Long Journey of Pontine Nuclei Neurons: From Rhombic Lip to Cortico-Ponto-Cerebellar Circuitry Claudius F. Kratochwil 1,2, Upasana Maheshwari 3,4 and Filippo M. Rijli 3,4* 1Chair in Zoology and Evolutionary Biology, Department of Biology, University of Konstanz, Konstanz, Germany, 2Zukunftskolleg, University of Konstanz, Konstanz, Germany, 3Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland, 4University of Basel, Basel, Switzerland The pontine nuclei (PN) are the largest of the precerebellar nuclei, neuronal assemblies in the hindbrain providing principal input to the cerebellum. The PN are predominantly innervated by the cerebral cortex and project as mossy fibers to the cerebellar hemispheres. Here, we comprehensively review the development of the PN from specification to migration, nucleogenesis and circuit formation. PN neurons originate at the posterior rhombic lip and migrate tangentially crossing several rhombomere derived territories to reach their final position in ventral part of the pons. The developing PN provide a classical example of tangential neuronal migration and a study system for understanding its molecular underpinnings. We anticipate that understanding the mechanisms of PN migration and assembly will also permit a deeper understanding of the molecular and cellular basis of cortico-cerebellar circuit formation and function. Keywords: pontine gray nuclei, reticulotegmental nuclei, precerebellar system, cortico-ponto-cerebellar circuitry, Hox genes Edited by: Takao K. Hensch, INTRODUCTION Harvard University, United States Reviewed by: The basal pontine nuclei (BPN) (also known as basilar pons, pontine gray nuclei or pontine nuclei Masahiko Takada, (PN)) and the reticulotegmental nuclei (RTN) (also known as nucleus reticularis tegmenti pontis) Kyoto University, Japan are located within the ventral portion of the pons.