The Long Journey of Pontine Nuclei Neurons : from Rhombic Lip To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -
Brainstem: Midbrainmidbrain
Brainstem:Brainstem: MidbrainMidbrain 1.1. MidbrainMidbrain –– grossgross externalexternal anatomyanatomy 2.2. InternalInternal structurestructure ofof thethe midbrain:midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain MidbrainMidbrain –– generalgeneral featuresfeatures location – between forebrain and hindbrain the smallest region of the brainstem – 6-7g the shortest brainstem segment ~ 2 cm long least differentiated brainstem division human midbrain is archipallian – shared general architecture with the most ancient of vertebrates embryonic origin – mesencephalon main functions:functions a sort of relay station for sound and visual information serves as a nerve pathway of the cerebral hemispheres controls the eye movement involved in control of body movement Prof. Dr. Nikolai Lazarov 2 Midbrain MidbrainMidbrain –– grossgross anatomyanatomy dorsal part – tectum (quadrigeminal plate): superior colliculi inferior colliculi cerebral aqueduct ventral part – cerebral peduncles:peduncles dorsal – tegmentum (central part) ventral – cerebral crus substantia nigra Prof. Dr. Nikolai Lazarov 3 Midbrain CerebralCerebral cruscrus –– internalinternal structurestructure CerebralCerebral peduncle:peduncle: crus cerebri tegmentum mesencephali substantia nigra two thick semilunar white matter bundles composition – somatotopically arranged motor tracts: corticospinal } pyramidal tracts – medial ⅔ corticobulbar corticopontine fibers: frontopontine tracts – medially temporopontine tracts – laterally -

DR. Sanaa Alshaarawy
By DR. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal, mid “Trigeminal level” and rostral). 3. Mid brain ( superior and inferior colliculi). Describe the Reticular formation (structure, function and pathway) being an important content of the brain stem. 2 1. Traversed by the Central Canal. Motor Decussation*. Spinal Nucleus of Trigeminal (Trigeminal sensory nucleus)* : ➢ It is a larger sensory T.S of Caudal part of M.O. nucleus. ➢ It is the brain stem continuation of the Substantia Gelatinosa of spinal cord 3 The Nucleus Extends : Through the whole length of the brain stem and upper segments of spinal cord. It lies in all levels of M.O, medial to the spinal tract of the trigeminal. It receives pain and temperature from face, forehead. Its tract present in all levels of M.O. is formed of descending fibers that terminate in the trigeminal nucleus. 4 It is Motor Decussation. Formed by pyramidal fibers, (75-90%) cross to the opposite side They descend in the Decuss- = crossing lateral white column of the spinal cord as the lateral corticospinal tract. The uncrossed fibers form the ventral corticospinal tract. 5 Traversed by Central Canal. Larger size Gracile & Cuneate nuclei, concerned with proprioceptive deep sensations of the body. Axons of Gracile & Cuneate nuclei form the internal arcuate fibers; decussating forming Sensory Decussation. Pyramids are prominent ventrally. 6 Formed by the crossed internal arcuate fibers Medial Leminiscus: Composed of the ascending internal arcuate fibers after their crossing. -

Barhl1regulates Migration and Survival of Cerebellar Granule
3104 • The Journal of Neuroscience, March 24, 2004 • 24(12):3104–3114 Development/Plasticity/Repair Barhl1 Regulates Migration and Survival of Cerebellar Granule Cells by Controlling Expression of the Neurotrophin-3 Gene Shengguo Li,1 Feng Qiu,1 Anlong Xu,2 Sandy M. Price,1 and Mengqing Xiang1 1Center for Advanced Biotechnology and Medicine and Department of Pediatrics, University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, Piscataway, New Jersey 08854, and 2Department of Biochemistry, College of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China The neurons generated at the germinal rhombic lip undergo long distance migration along divergent pathways to settle in widely dispersed locations within the hindbrain, giving rise to cerebellar granule cells and precerebellar nuclei. Neurotrophin-3 (NT-3) signaling has been shown to be required for proper migration and survival of cerebellar granule cells. The molecular bases that govern NT-3 expression within the cerebellum, however, remain unknown at present. Here we report that, during early mouse neurogenesis, the Barhl1 homeobox gene is highly expressed by the rhombic lip and rhombic lip-derived migratory neurons. Its expression is later restricted to cerebellar granule cells and precerebellar neurons extending mossy fibers, two groups of neurons that synaptically connect in the adult cerebellar system. Loss of Barhl1 function causes cerebellar phenotypes with a striking similarity to those of NT-3 conditional null mice, which include attenuated cerebellar foliation as well as defective radial migration and increased apoptotic death of granule cells. Correlating with these defects, we find that NT-3 expression is dramatically downregulated in granule cells of the posterior lobe of Ϫ Ϫ Ϫ Ϫ Barhl1 / cerebella. -
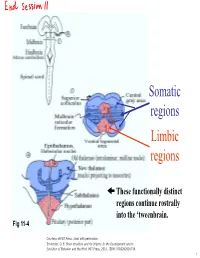
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

Brainstem and Its Associated Cranial Nerves
Brainstem and its Associated Cranial Nerves Anatomical and Physiological Review By Sara Alenezy With appreciation to Noura AlTawil’s significant efforts Midbrain (Mesencephalon) External Anatomy of Midbrain 1. Crus Cerebri (Also known as Basis Pedunculi or Cerebral Peduncles): Large column of descending “Upper Motor Neuron” fibers that is responsible for movement coordination, which are: a. Frontopontine fibers b. Corticospinal fibers Ventral Surface c. Corticobulbar fibers d. Temporo-pontine fibers 2. Interpeduncular Fossa: Separates the Crus Cerebri from the middle. 3. Nerve: 3rd Cranial Nerve (Oculomotor) emerges from the Interpeduncular fossa. 1. Superior Colliculus: Involved with visual reflexes. Dorsal Surface 2. Inferior Colliculus: Involved with auditory reflexes. 3. Nerve: 4th Cranial Nerve (Trochlear) emerges caudally to the Inferior Colliculus after decussating in the superior medullary velum. Internal Anatomy of Midbrain 1. Superior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). a. Tectospinal Pathway: turning the head, neck and eyeballs in response to a visual stimuli.1 Level of b. Spinotectal Pathway: turning the head, neck and eyeballs in response to a cutaneous stimuli.2 Superior 2. Oculomotor Nucleus: Situated in the periaqueductal grey matter. Colliculus 3. Red Nucleus: Red mass3 of grey matter situated centrally in the Tegmentum. Involved in motor control (Rubrospinal Tract). 1. Inferior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). Tectospinal Pathway: turning the head, neck and eyeballs in response to a auditory stimuli. 2. Trochlear Nucleus: Situated in the periaqueductal grey matter. Level of Inferior 3. -

ON-LINE FIG 1. Selected Images of the Caudal Midbrain (Upper Row
ON-LINE FIG 1. Selected images of the caudal midbrain (upper row) and middle pons (lower row) from 4 of 13 total postmortem brains illustrate excellent anatomic contrast reproducibility across individual datasets. Subtle variations are present. Note differences in the shape of cerebral peduncles (24), decussation of superior cerebellar peduncles (25), and spinothalamic tract (12) in the midbrain of subject D (top right). These can be attributed to individual anatomic variation, some mild distortion of the brain stem during procurement at postmortem examination, and/or differences in the axial imaging plane not easily discernable during its prescription parallel to the anterior/posterior commissure plane. The numbers in parentheses in the on-line legends refer to structures in the On-line Table. AJNR Am J Neuroradiol ●:●●2019 www.ajnr.org E1 ON-LINE FIG 3. Demonstration of the dentatorubrothalamic tract within the superior cerebellar peduncle (asterisk) and rostral brain stem. A, Axial caudal midbrain image angled 10° anterosuperior to posteroinferior relative to the ACPC plane demonstrates the tract traveling the midbrain to reach the decussation (25). B, Coronal oblique image that is perpendicular to the long axis of the hippocam- pus (structure not shown) at the level of the ventral superior cerebel- lar decussation shows a component of the dentatorubrothalamic tract arising from the cerebellar dentate nucleus (63), ascending via the superior cerebellar peduncle to the decussation (25), and then enveloping the contralateral red nucleus (3). C, Parasagittal image shows the relatively long anteroposterior dimension of this tract, which becomes less compact and distinct as it ascends toward the thalamus. ON-LINE FIG 2. -

Exploring Prefrontal Cortical Memory Mechanisms with Eyeblink Conditioning
Behavioral Neuroscience © 2011 American Psychological Association 2011, Vol. 125, No. 3, 318–326 0735-7044/11/$12.00 DOI: 10.1037/a0023520 Exploring Prefrontal Cortical Memory Mechanisms With Eyeblink Conditioning Craig Weiss and John F. Disterhoft Northwestern University Feinberg School of Medicine Several studies in nonhuman primates have shown that neurons in the dorsolateral prefrontal cortex have activity that persists throughout the delay period in delayed matching to sample tasks, and age-related changes in the microcolumnar organization of the prefrontal cortex are significantly correlated with age-related declines in cognition. Activity that persists beyond the presentation of a stimulus could mediate working memory processes, and disruption of those processes could account for memory deficits that often accompany the aging process. These potential memory and aging mechanisms are being systematically examined with eyeblink conditioning paradigms in nonprimate mammalian animal models including the rabbit. The trace version of the conditioning paradigm is a particularly good system to explore declarative memory since humans do not acquire trace conditioning if they are unable to become cognitively aware of the association between a conditioning tone and an airpuff to the eye. This conditioning paradigm has been used to show that the hippocampus and cerebellum interact functionally since both conditioned responses and conditioned hippocampal pyramidal neuron activity are abolished following lesions of the cerebellar nuclei and since hippocampal lesions prevent or abolish trace conditioned blinks. However, because there are no direct connections between the hippocampal forma- tion and the cerebellum, and because the hippocampus is not necessary for trace conditioning after a period of consolidation has elapsed, we and others have been examining the prefrontal cortex for its role in forebrain-dependent trace eyeblink conditioning. -

Focal Projections of Cat Auditory Cortex to the Pontine Nuclei
THE JOURNAL OF COMPARATIVE NEUROLOGY 497:959–980 (2006) Focal Projections of Cat Auditory Cortex to the Pontine Nuclei 1 2 1 MERCEDES PERALES, JEFFERY A. WINER, AND JORGE J. PRIETO * 1Department of Histology and Anatomy, University Miguel Hernandez, 03550 — Sant Joan d’Alacant, Spain 2Division of Neurobiology, Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, California 94720-3200 ABSTRACT The pontine nuclei (PN) receive projections from the auditory cortex (AC) and they are a major source of mossy fibers to the cerebellum. However, they have not been studied in detail using sensitive neuroanatomical tracers, and whether all AC areas contribute to the corti- copontine (CP) system is unknown. We characterized the projection patterns of 11 AC areas with WGA-HRP. We also compared them with their corticothalamic and corticocollicular counterparts. A third objective was to analyze the structure of the CP axons and their terminals with BDA. Both tracers confirm that all AC areas projected to lateral, central, and medial ipsilateral pontine divisions. The strongest CP projections were from nontonotopic and polymodal association areas. Preterminal fibers formed single terminal fields having many boutons en passant as well as terminal endings, and there was a specific morphological pattern for each pontine target, irrespective of their areal origin. Thus, axons in the medial division had a simpler terminal architecture (type 1 terminal plexus); both the central and lateral pons received more complex endings (type 2 terminal plexus). Auditory CP topograph- ical distribution resembled visual and somatosensory CP projections, which preserve retino- topy and somatotopy in the pons, respectively. -

Cerebellar Cortical Neuron Responses Evoked from the Spinal Border Cell Tract
Cerebellar cortical neuron responses evoked from the spinal border cell tract. Geborek, Pontus; Spanne, Anton; Bengtsson, Fredrik; Jörntell, Henrik Published in: Frontiers in Neural Circuits DOI: 10.3389/fncir.2013.00157 2013 Link to publication Citation for published version (APA): Geborek, P., Spanne, A., Bengtsson, F., & Jörntell, H. (2013). Cerebellar cortical neuron responses evoked from the spinal border cell tract. Frontiers in Neural Circuits, 7, [157]. https://doi.org/10.3389/fncir.2013.00157 Total number of authors: 4 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 04. Oct. 2021 ORIGINAL RESEARCH ARTICLE published: 08 October 2013 NEURAL CIRCUITS doi: 10.3389/fncir.2013.00157 Cerebellar cortical neuron responses evoked from the spinal border cell tract Pontus Geborek, Anton Spanne, Fredrik Bengtsson and Henrik Jörntell* Neural Basis of Sensorimotor Control, Department of Experimental Medical Science, Lund University, Lund, Sweden Edited by: Spinocerebellar systems are likely to be crucial for cerebellar hallmark functions such Egidio D’Angelo, University of Pavia, as coordination. -

Impaired Cerebro-Cerebellar White Matter Connectivity and Its
www.nature.com/npjschz ARTICLE OPEN Impaired cerebro-cerebellar white matter connectivity and its associations with cognitive function in patients with schizophrenia ✉ Sung Eun Kim1, Sungcheol Jung2, Gyhye Sung1,3, Minji Bang 1 and Sang-Hyuk Lee1 Schizophrenia is a complex brain disorder of unknown etiology. Based on the notion of “cognitive dysmetria,” we aimed to investigate aberrations in structural white matter (WM) connectivity that links the cerebellum to cognitive dysfunction in patients with schizophrenia. A total of 112 participants (65 patients with schizophrenia and 47 healthy controls [HCs]) were enrolled and underwent diffusion tensor imaging. Between-group voxel-wise comparisons of cerebellar WM regions (superior/middle [MCP]/ inferior cerebellar peduncle and pontine crossing fibers) were performed using Tract-Based Spatial Statistics. Cognitive function was assessed using the Trail Making Test Part A/B (TMT-A/B), Wisconsin Card Sorting Test (WCST), and Rey-Kim Memory Test in 46 participants with schizophrenia. WM connectivity, measured as fractional anisotropy (FA), was significantly lower in the MCP in participants with schizophrenia than in HCs. The mean FAs extracted from the significant MCP cluster were inversely correlated with poorer cognitive performance, particularly longer time to complete the TMB-B (r = 0.559, p < 0.001) and more total errors in the WCST (r = 0.442, p = 0.003). Our findings suggest that aberrant cerebro-cerebellar communication due to disrupted WM connectivity may contribute to cognitive impairments, a core characteristic of schizophrenia. Our results may expand our 1234567890():,; understanding of the neurobiology of schizophrenia based on the cerebro-cerebellar interconnectivity of the brain. npj Schizophrenia (2021) 7:38 ; https://doi.org/10.1038/s41537-021-00169-w INTRODUCTION patients with schizophrenia, implying the possible involvement of Schizophrenia is a complex brain disorder of unknown etiology.