Locked-In- Syndrome (Aka
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -
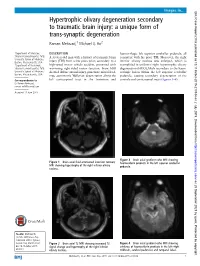
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

Closed-Loop Neuromodulation Restores Network Connectivity And
RESEARCH ARTICLE Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury Patrick D Ganzer1,2*, Michael J Darrow1, Eric C Meyers1,2, Bleyda R Solorzano2, Andrea D Ruiz2, Nicole M Robertson2, Katherine S Adcock3, Justin T James2, Han S Jeong2, April M Becker4, Mark P Goldberg4, David T Pruitt1,2, Seth A Hays1,2,3, Michael P Kilgard1,2,3, Robert L Rennaker II1,2,3* 1Erik Jonsson School of Engineering and Computer Science, The University of Texas at Dallas, Richardson, United States; 2Texas Biomedical Device Center, Richardson, United States; 3School of Behavioral Brain Sciences, The University of Texas at Dallas, Richardson, United States; 4Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, United States Abstract Recovery from serious neurological injury requires substantial rewiring of neural circuits. Precisely-timed electrical stimulation could be used to restore corrective feedback mechanisms and promote adaptive plasticity after neurological insult, such as spinal cord injury (SCI) or stroke. This study provides the first evidence that closed-loop vagus nerve stimulation (CLV) based on the synaptic eligibility trace leads to dramatic recovery from the most common forms of SCI. The addition of CLV to rehabilitation promoted substantially more recovery of forelimb function compared to rehabilitation alone following chronic unilateral or bilateral cervical SCI in a rat model. Triggering stimulation on the most successful movements is critical to maximize recovery. CLV enhances recovery by strengthening synaptic connectivity from remaining motor *For correspondence: networks to the grasping muscles in the forelimb. The benefits of CLV persist long after the end of [email protected] stimulation because connectivity in critical neural circuits has been restored. -

Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: a Study Using a High-Resolution Semiconductor PET System
Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: A Study Using a High-Resolution Semiconductor PET System Kenji Hirata1,2, Naoya Hattori3, Wataru Takeuchi4, Tohru Shiga1, Yuichi Morimoto4, Kikuo Umegaki5, Kentaro Kobayashi1, Osamu Manabe1, Shozo Okamoto1, and Nagara Tamaki1 1Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA, Los Angeles, California; 3Department of Molecular Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 4Research and Development Group, Hitachi Ltd., Tokyo, Japan; and 5Division of Quantum Science and Engineering, Faculty of Engineering, Hokkaido University, Sapporo, Japan (1). The rubrospinal tract mainly controls limb musculature, whereas The red nucleus (RN) is a pair of small gray matter structures located the pyramidal tract can act on the whole musculature (1). A recent in the midbrain and involved in muscle movement and cognitive anatomic study indicated that a significant amount of rubrospinal tract functions. This retrospective study aimed to investigate the metabo- is present in the human brain (2). Because neuronal activity of the RN lism of human RN and its correlation to other brain regions. Methods: in Parkinson disease is known to be increased during passive and We developed a high-resolution semiconductor PET system to image voluntary movements (3), the RN may play a role in the coordination small brain structures. Twenty patients without neurologic disorders of muscular movement. Cognitive symptoms, such as intellectual underwent whole-brain scanning after injection of 400 MBq of fatigability, decreased verbal fluency, and discrete memory impair- 18F-FDG. -

Orienting Head Movements Resulting from Electrical Microstimulation of the Brainstem Tegmentum in the Barn Owl
The Journal of Neuroscience, January 1993, 13(l): 351370 Orienting Head Movements Resulting from Electrical Microstimulation of the Brainstem Tegmentum in the Barn Owl Tom Masino and Eric I. Knudsen Department of Neurobiology, Stanford University, Stanford, California 943055401 The size and direction of orienting movements are repre- movement latency, duration, velocity, and size each dem- sented systematically as a motor map in the optic tectum of onstrated dependencies on stimulus amplitude, frequency, the barn owl (du Lac and Knudsen, 1990). The optic tectum and duration. projects to several distinct regions in the medial brainstem The data demonstrate directly that at the level of the mid- tegmentum, which in turn project to the spinal cord (Masino brain tegmentum there exists a three-dimensional Cartesian and Knudsen, 1992). This study explores the hypothesis that representation of head-orienting movements such that hor- a fundamental transformation in the neural representation izontal, vertical, and roll components of movement are en- of orienting movements takes place in the brainstem teg- coded by anatomically distinct neural circuits. The data sug- mentum. Head movements evoked by electrical microstim- gest that in the projection from the optic tectum to these ulation in the brainstem tegmentum of the alert barn owl were medial tegmental regions, the topographic code for orienting cataloged and the sites of stimulation were reconstructed movement that originates in the tectum is transformed into histologically. Movements elicited from the brainstem teg- this Cartesian code. mentum were categorized into one of six different classes: [Key words: optic tectum, superior colliculus, saccadic saccadic head rotations, head translations, facial move- head movement, brainstem tegmentum, interstitial nucleus ments, vocalizations, limb movements, and twitches. -

Optogenetic Activation of Cholinergic Neurons in the PPT Or LDT Induces REM Sleep
Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep Christa J. Van Dorta,b,c,1, Daniel P. Zachsa,b,c, Jonathan D. Kennya,b,c, Shu Zhengb, Rebecca R. Goldblumb,c,d, Noah A. Gelwana,b,c, Daniel M. Ramosb,c, Michael A. Nolanb,c,d, Karen Wangb,c, Feng-Ju Wengb,e, Yingxi Linb,e, Matthew A. Wilsonb,c, and Emery N. Browna,b,d,f,1 aDepartment of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114; and bDepartment of Brain and Cognitive Sciences, cPicower Institute for Learning and Memory, eMcGovern Institute for Brain Research, fHarvard-MIT Division of Health Sciences and Technology, and dInstitute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Emery N. Brown, December 3, 2014 (sent for review September 19, 2014; reviewed by Helen A. Baghdoyan and H. Craig Heller) Rapid eye movement (REM) sleep is an important component of REM sleep regulation, a method that can modulate specific cell the natural sleep/wake cycle, yet the mechanisms that regulate types in the behaving animal is needed. Optogenetics now pro- REM sleep remain incompletely understood. Cholinergic neurons vides this ability to target specific subpopulations of neurons in the mesopontine tegmentum have been implicated in REM sleep and control them with millisecond temporal resolution (30). regulation, but lesions of this area have had varying effects on REM Therefore, we aimed to determine the role of cholinergic sleep. Therefore, this study aimed to clarify the role of cholinergic neurons in the PPT and LDT in REM sleep regulation using neurons in the pedunculopontine tegmentum (PPT) and laterodor- optogenetics. -

Brainstem Dental 2012.Doc
Dental Neuroanatomy January 12 and 19, 10-12, 2012 Suzanne S. Stensaas, Ph.D. Dear Students: Please print these notes and bring them with you. My style is to use a Tablet PC and I draw on either a Word or pdf copy with colors. Be prepared to draw. Have at least 5 colors. Please try to look at the notes AHEAD OF TIME for each lecture in this course. This way you can see the direction and organization of the lecture and be more familiar with the terms. There will be a quiz (that does not count) at the beginning to cover topics in the two gross anatomy lectures by Dr. Morton in Phase 1. They are G 17B and GL 18 Waxman, S Clinical Neuroanatomy, 26th ed.2010. THE OLD EDITION IS FINE TOO. Review Ch 5 on the spinal cord organization, but not the tracts in the middle or lesions at the end of the chapter. Also review the basic concept of a reflex. Review or skim Ch 12 on the vascular supply of the brain. Just look at pictures and legends for the clinical part at the end. NEW material: Chapter 7 Waxman, Brainstem, but not the cerebellum part. NEW material: Chapter 8 Waxman, Cranial nerves, all of it including autonomic. BEWARE THE CRANIAL NERVES ARE KILLERS! There are about 50 copies of the following bright yellow paperback book, which can be checked out from the Eccles Health Sciences Library and kept for the duration of the course. They are on reserve as: Cranial nerves: anatomy and clinical comments Linda Wilson-Pauwels, 1988 Toronto; Philadelphia: B.C. -

Isolated Necrosis of Central Tegmental Tracts Due to Neonatal Hypoxic-Ischemic Encephalopathy: MRI Findings
Journal of Neurology & Stroke Case Report Open Access Isolated necrosis of central tegmental tracts due to neonatal hypoxic-ischemic encephalopathy: MRI findings Abstract Volume 11 Issue 1 - 2021 Perinatal hypoxia is an old entity that still prevails today and may lead to neurological Tomás de Andrade Lourenção Freddi, Luiz sequelae that can go unnoticed until a certain age, generating many costs for public health. In this case report, we demonstrate on magnetic resonance imaging an unusual pattern of Fellipe Curvêlo Ciraulo Santos, Nelson Paes perinatal hypoxia in a preterm 5-month-old infant, involving the central tegmental tracts Fortes Diniz Ferreira, Felipe Diego Gomes and briefly discuss its possible pathophysiology. Dantas Department of Radiology, Hospital do Coração, Brazil Keywords: magnetic resonance imaging, asphyxia, hypoxic-ischemic encephalopathy, tegmentum, neonates, brainstem Correspondence: Tomás de Andrade Lourenção Freddi, Hospital do Coração, 147 Desembargador Eliseu Guilherme Street, São Paulo, SP, 04004-030, Brazil, Tel +5511976059280, Email Received: May 25, 2020 | Published: Febrauary 15, 2021 Abbreviations: MRI, magnetic resonance imaging; HII, that connect the red nucleus and the inferior olivary nucleus, being hypoxic-ischemic injury; FLAIR, fluid-attenuated inversion recovery; part of the dentato-rubro-olivary system, called Guillain–Mollaret CTT, central tegmental tract; VGB, vigabatrin triangle.3–5 Introduction Hypoxic-ischemic injury (HII) is one of the most important causes of encephalopathy in neonates, irrespective of gestational age, and may occur in the uterus or during delivery by different intrapartum conditions. In preterm or very low birth weight infants, brain magnetic resonance imaging (MRI) can demonstrate multiple different findings, which a detailed description is beyond the scope of this article, although being periventricular leukomalacia the most frequent (seen in at least 50% of cases). -

Chapter 128: Basic Mechanisms of Sleep: New Evidence On
128 BASIC MECHANISMS OF SLEEP: NEW EVIDENCE ON THE NEUROANATOMY AND NEUROMODULATION OF THE NREM-REM CYCLE EDWARD F. PACE-SCHOTT J. ALLAN HOBSON The 1990s brought a wealth of new detail to our knowledge NREM sleep (noradrenergic, serotonergic, and cholinergic of the brain structures involved in the control of sleep and systems damped), and REM sleep (noradrenergic and sero- waking and in the cellular level mechanisms that orchestrate tonergic systems off, cholinergic system undamped) (1–4). the sleep cycle through neuromodulation. This chapter pre- sents these new findings in the context of the general history Original Reciprocal Interaction Model: An of research on the brainstem neuromodulatory systems and Aminergic-Cholinergic Interplay the more specific organization of those systems in the con- trol of the alternation of wake, non–rapid eye movement The model of reciprocal interaction (5) provided a theoretic (NREM), and REM sleep. framework for experimental interventions at the cellular and Although the main focus of the chapter is on the our molecular level that has vindicated the notion that waking own model of reciprocal aminergic-cholinergic interaction, and REM sleep are at opposite ends of an aminergically we review new data suggesting the involvement of many dominant to cholinergically dominant neuromodulatory more chemically specific neuronal groups than can be ac- continuum, with NREM sleep holding an intermediate po- commodated by that model. We also extend our purview to sition (Fig. 128.1). The reciprocal interaction hypothesis the way in which the brainstem interacts with the forebrain. (5) provided a description of the aminergic-cholinergic in- These considerations inform not only sleep-cycle control terplay at the synaptic level and a mathematic analysis of per se, but also the way that circadian and ultradian rhythms the dynamics of the neurobiological control system. -

Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
The Journal of Neuroscience, September 1, 2000, 20(17):6561–6569 Compensatory Sprouting and Impulse Rerouting after Unilateral Pyramidal Tract Lesion in Neonatal Rats Werner J. Z’Graggen, Karim Fouad, Olivier Raineteau, Gerlinde A. S. Metz, Martin E. Schwab, and Gwendolyn L. Kartje Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8057 Zurich, Switzerland After lesions of the developing mammalian CNS, structural plas- tigate possible new functional connections from the motor cortex ticity and functional recovery are much more pronounced than in of the pyramidotomy side to the periphery. In rats lesioned as the mature CNS. We investigated the anatomical reorganization adults, stimulation of the motor cortex ipsilateral to the pyra- of the corticofugal projections rostral to a unilateral lesion of the midotomy never elicited EMG activity. In contrast, in P2 lesioned corticospinal tract at the level of the medullary pyramid (pyra- rats bilateral forelimb EMGs were found. EMG latencies were midotomy) and the contribution of this reorganization and other comparable for the ipsilateral and contralateral responses but descending systems to functional recovery. were significantly longer than in unlesioned animals. Transient Two-day-old (P2) and adult rats underwent a unilateral pyra- inactivation of both red nuclei with the GABA receptor agonist midotomy. Three months later the corticofugal projections to the muscimol led to a complete loss of these bilateral movements. red nucleus and the pons were analyzed; a relatively large num- Movements and EMGs reappeared after wash-out of the drug. ber of corticorubral and corticopontine fibers from the lesioned These results suggest an important role of the red nucleus in the side had crossed the midline and established an additional con- tralateral innervation of the red nucleus and the pons. -

Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study
Original Article J. Phys. Ther. Sci. 22: 7–10, 2010 Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study SUNG HO JANG, MD1), JI HEON HONG, PT, MS1), YONG HYUN KWON, PT, PhD2) 1)Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 2)Department of Physical Therapy, Yeungnam College of Science & Technology: 1737, Daemyung 7-Dong, Namgu, Daegu, 705-703, Republic of Korea. TEL +82 53-650-9702, FAX: +82 53-629-5048 Abstract. [Purpose] Little is known about the rubro-olivary tract (ROT) in the human brain. We attempted to identify the ROT using diffusion tensor tractography (DTT). [Subjects and Methods] We obtained ROT data from 11 healthy subjects with no history of neurological disorder. For tracking of the ROT, a seed region of interest (ROI) was selected in the red nucleus, and a target ROI was found in the inferior olive of each subject. [Results] The ROT, originated in the red nucleus, and passed laterally to the decussation of the superior cerebellar peduncle in the lower midbrain. In the pons, it descended through the area adjacent to the medial lemnicus in the posterior direction. Within the medulla, the ROT ended in the inferior olive, which was located lateral to the medial lemnicus and posterior to the pyramid. [Conclusion] We identified the ROT in the human brain using DTT. These results will be informative for research into the ROT in the human brain. Key words: Diffusion tensor image, Red nucleus, Inferior olive (This article was submitted Jul. 24, 2009, and was accepted Sep.