White Matter Dissection and Structural Connectivity of the Human Vertical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Combined Structural and Diffusion Tensor Imaging Detection of Ischemic Injury in Moyamoya Disease: Relation to Disease Advancement and Cerebral Hypoperfusion
CLINICAL ARTICLE Combined structural and diffusion tensor imaging detection of ischemic injury in moyamoya disease: relation to disease advancement and cerebral hypoperfusion Ken Kazumata, MD, PhD,1 Kikutaro Tokairin, MD,1 Masaki Ito, MD, PhD,1 Haruto Uchino, MD, PhD,1 Taku Sugiyama, MD, PhD,1 Masahito Kawabori, MD, PhD,1 Toshiya Osanai, MD, PhD,1 Khin Khin Tha, MD, PhD,2 and Kiyohiro Houkin, MD, PhD1 1Department of Neurosurgery, Hokkaido University Graduate School of Medicine; and 2Clinical Research and Medical Innovation Center, Hokkaido University Hospital, Sapporo, Japan OBJECTIVE The microstructural integrity of gray and white matter is decreased in adult moyamoya disease, suggesting covert ischemic injury as a mechanism of cognitive dysfunction. Establishing a microstructural brain imaging marker is critical for monitoring cognitive outcomes following surgical interventions. The authors of the present study determined the pathophysiological basis of altered microstructural brain injury in relation to advanced arterial occlusion, cerebral hypoperfusion, and cognitive function. METHODS The authors examined 58 patients without apparent brain lesions and 30 healthy controls by using structural MRI, as well as diffusion tensor imaging (DTI). Arterial occlusion in each hemisphere was classified as early or ad- vanced stage based on MRA and posterior cerebral artery (PCA) involvement. Regional cerebral blood flow (rCBF) was measured with N-isopropyl-p-[123I]-iodoamphetamine SPECT. Furthermore, cognitive performance was examined using the Wechsler Adult Intelligence Scale, Third Edition and the Trail Making Test (TMT). Both voxel- and region of inter- est–based analyses were performed for groupwise comparisons, as well as correlation analysis, using parameters such as cognitive test scores; gray matter volume; fractional anisotropy (FA) of association fiber tracts, including the inferior frontooccipital fasciculus (IFOF) and superior longitudinal fasciculus (SLF); PCA involvement; and rCBF. -

Circular Representation of Human Cortical Networks for Subject and Population-Level Connectomic Visualization
NeuroImage 60 (2012) 1340–1351 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Full Length Articles Circular representation of human cortical networks for subject and population-level connectomic visualization Andrei Irimia ⁎, Micah C. Chambers, Carinna M. Torgerson, John D. Van Horn Laboratory of Neuro Imaging, Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles, 635 Charles E Young Drive South, Suite 225, Los Angeles, CA 90095, USA article info abstract Article history: Cortical network architecture has predominantly been investigated visually using graph theory representa- Received 13 September 2011 tions. In the context of human connectomics, such representations are not however always satisfactory Revised 19 January 2012 because canonical methods for vertex–edge relationship representation do not always offer optimal insight Accepted 20 January 2012 regarding functional and structural neural connectivity. This article introduces an innovative framework for Available online 28 January 2012 the depiction of human connectomics by employing a circular visualization method which is highly suitable to the exploration of central nervous system architecture. This type of representation, which we name a Keywords: ‘ ’ Connectomics connectogram , has the capability of classifying neuroconnectivity relationships intuitively and elegantly. A Cortical network multimodal protocol for MRI/DTI neuroimaging data acquisition is here combined with automatic image seg- DTI mentation to (1) extract cortical and non-cortical anatomical structures, (2) calculate associated volumetrics MRI and morphometrics, and (3) determine patient-specific connectivity profiles to generate subject-level and Neuroimaging population-level connectograms. The scalability of our approach is demonstrated for a population of 50 adults. -

Quantitative Analysis of Axon Collaterals of Single Pyramidal Cells
Yang et al. BMC Neurosci (2017) 18:25 DOI 10.1186/s12868-017-0342-7 BMC Neuroscience RESEARCH ARTICLE Open Access Quantitative analysis of axon collaterals of single pyramidal cells of the anterior piriform cortex of the guinea pig Junli Yang1,2*, Gerhard Litscher1,3* , Zhongren Sun1*, Qiang Tang1, Kiyoshi Kishi2, Satoko Oda2, Masaaki Takayanagi2, Zemin Sheng1,4, Yang Liu1, Wenhai Guo1, Ting Zhang1, Lu Wang1,3, Ingrid Gaischek3, Daniela Litscher3, Irmgard Th. Lippe5 and Masaru Kuroda2 Abstract Background: The role of the piriform cortex (PC) in olfactory information processing remains largely unknown. The anterior part of the piriform cortex (APC) has been the focus of cortical-level studies of olfactory coding, and asso- ciative processes have attracted considerable attention as an important part in odor discrimination and olfactory information processing. Associational connections of pyramidal cells in the guinea pig APC were studied by direct visualization of axons stained and quantitatively analyzed by intracellular biocytin injection in vivo. Results: The observations illustrated that axon collaterals of the individual cells were widely and spatially distrib- uted within the PC, and sometimes also showed a long associational projection to the olfactory bulb (OB). The data showed that long associational axons were both rostrally and caudally directed throughout the PC, and the intrinsic associational fibers of pyramidal cells in the APC are omnidirectional connections in the PC. Within the PC, associa- tional axons typically followed rather linear trajectories and irregular bouton distributions. Quantitative data of the axon collaterals of two pyramidal cells in the APC showed that the average length of axonal collaterals was 101 mm, out of which 79 mm (78% of total length) were distributed in the PC. -

Progress and Challenges in Probing the Human Brain
University of Pennsylvania ScholarlyCommons Neuroethics Publications Center for Neuroscience & Society 10-2015 Progress and Challenges in Probing the Human Brain Russell A. Poldrack Martha J. Farah University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/neuroethics_pubs Part of the Bioethics and Medical Ethics Commons, Neuroscience and Neurobiology Commons, and the Neurosciences Commons Recommended Citation Poldrack, R. A., & Farah, M. J. (2015). Progress and Challenges in Probing the Human Brain. Nature, 526 (7573), 371-379. http://dx.doi.org/10.1038/nature15692 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/neuroethics_pubs/136 For more information, please contact [email protected]. Progress and Challenges in Probing the Human Brain Abstract Perhaps one of the greatest scientific challenges is to understand the human brain. Here we review current methods in human neuroscience, highlighting the ways that they have been used to study the neural bases of the human mind. We begin with a consideration of different levels of description relevant to human neuroscience, from molecules to large-scale networks, and then review the methods that probe these levels and the ability of these methods to test hypotheses about causal mechanisms. Functional MRI is considered in particular detail, as it has been responsible for much of the recent growth of human neuroscience research. We briefly er view its inferential strengths and weaknesses and present examples of new analytic approaches that allow inferences beyond simple localization of psychological processes. Finally, we review the prospects for real-world applications and new scientific challenges for human neuroscience. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Evolutionary Expansion of Connectivity Between Multimodal Association Areas in the Human Brain Compared with Chimpanzees,” by Dirk Jan Ardesch, Lianne H
Correction NEUROSCIENCE Correction for “Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees,” by Dirk Jan Ardesch, Lianne H. Scholtens, Longchuan Li, Todd M. Preuss, James K. Rilling, and Martijn P. van den Heuvel, which was first published March 18, 2019; 10.1073/pnas.1818512116 (Proc Natl Acad Sci USA 116:7101–7106). The authors wish to note the following: “No new MRI data were acquired for this study. All chimpanzee MRIs were obtained from a data archive of scans obtained prior to the 2015 implementation of US Fish and Wildlife Service and National Institutes of Health regulations governing research with chimpanzees. All the scans reported in this publication were completed by the end of 2012. All chimpanzee MRI scans are part of the National Chimpanzee Brain Resource (http://www.chimpanzeebrain.org).” Published under the PNAS license. Published online April 29, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1906107116 9680 | PNAS | May 7, 2019 | vol. 116 | no. 19 www.pnas.org Downloaded by guest on October 1, 2021 Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees Dirk Jan Ardescha, Lianne H. Scholtensa, Longchuan Lib, Todd M. Preussc,d,e, James K. Rillingc,d,f,g,h, and Martijn P. van den Heuvela,i,1 aConnectome Lab, Department of Complex Trait Genetics, Center for Neurogenomics and Cognitive Research, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, 1081 HV Amsterdam, The Netherlands; bMarcus Autism Center, Children’s Healthcare of Atlanta, Emory University, Atlanta, GA 30329; cYerkes National Primate Research Center, Emory University, Atlanta, GA 30329; dCenter for Translational Social Neuroscience, Emory University, Atlanta, GA 30329; eDepartment of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA 30307; fDepartment of Anthropology, Emory University, Atlanta, GA 30322; gSilvio O. -

Acute Cognitive Deficits After Traumatic Brain Injury Predict Alzheimer's
GeroScience (2020) 42:1411–1429 https://doi.org/10.1007/s11357-020-00245-6 ORIGINAL ARTICLE Acute cognitive deficits after traumatic brain injury predict Alzheimer’s disease-like degradation of the human default mode network Andrei Irimia & Alexander S. Maher & Nikhil N. Chaudhari & Nahian F. Chowdhury & Elliot B. Jacobs & the Alzheimer’s Disease Neuroimaging Initiative Received: 9 June 2020 /Accepted: 29 July 2020 /Published online: 2 August 2020 # American Aging Association 2020 Abstract Traumatic brain injury (TBI) and Alzheimer’s are leveraged to study the extent to which geriatric mild disease (AD) are prominent neurological conditions whose TBI(mTBI)canleadtoAD-like alteration of resting-state neural and cognitive commonalities are poorly understood. activity in the default mode network (DMN). This network The extent of TBI-related neurophysiological abnormali- is found to contain modules whose extent of AD-like, ties has been hypothesized to reflect AD-like neurodegen- posttraumatic degradation can be accurately prognosticat- eration because TBI can increase vulnerability to AD. ed based on the acute cognitive deficits of geriatric mTBI However, it remains challenging to prognosticate AD risk patients with cerebral microbleeds. Aside from establish- partly because the functional relationship between acute ing a predictive physiological association between geriatric posttraumatic sequelae and chronic AD-like degradation mTBI, cognitive impairment, and AD-like functional deg- remains elusive. Here, functional magnetic resonance im- radation, these findings advance the goal of acutely fore- aging (fMRI), network theory, and machine learning (ML) casting mTBI patients’ chronic deviations from normality along AD-like functional trajectories. The association of Andrei Irimia and Alexander S. Maher contributed equally to this geriatric mTBI with AD-like changes in functional brain work. -

A Fiber, 9, 10, 66, 67 Abdomen, 221 Visceral Afferent, 222 Absolute
INDEX A fiber, 9, 10, 66, 67 all-or-none law, 62 Abdomen, 221 current during propagation, 62, 63 visceral afferent, 222 current loop, 64 Absolute temperature, 26 definition, 40 Acceleration depolarization phase, 38 angular, 183 duration, 38 linear, 183, 184 effect on contraction, 144 negative, 184 frequency, 50 positive, 184 generation, 88, 89 Accommodation, excitability, 60 inactivation, 48 Acetic acid, 74, 78 inhibition, 96 Acetylenoline ion current, 42, 43 cycle, 78 ion shift, 40, 42, 43 end plate, 74, 75 kinetics, 44-52 fate, 77, 78 mechanism of propagation, 62 intestinal muscle, 237 membrane conductance, 49 membrane receptor, 77-79 muscle, 129 muscarinergic transmission, 223, 225 overshoot, 38 nicotinergic transmission, 223, 225 peak, 38 quanta, 82 phase, 38 receptor, 78, 79 potassium conductance, 41 Renshaw cell, 100 propagation, 61-68 smooth muscle, 230, 231 refractory period, 50 transmitter function, 100, 101 refractory phase, 49, 50 Acetylcholinesterase, 101 repolarization, 38 ACh, 74, 75, s.a. acetylcholine rising phase, 38 Acid, fatty, 225 saltatory conduction, 64-66 Actin, 131-133, 139, 147 smooth muscle, 230, 231 Actinomycin, '312 sodium conductance, 41, 42 Action potential, 37-43 sodium deficiency, 43 Action potential tetrodotoxin, 52 after-potential, 39 threshold, 39 327 328 Index Action potential (cont.) Anion, 21 time course, 37, 38 Anococcygeal muscle, 233 trigger, 39 Anoce,58 triphasic current, 64 Antagonist inhibition, 109, 212 upstroke, 38 Anterior pens, micturition center, 241 velocity of conduction, 61 Anticholinergic substance, 313 Active transport Antidiuretic hormone, 259 membrane, 32 Aphagia, 264 sodium, 35 Aphasia Activity clock, 288 motor, 305 Adaptation, hormonal, 259 sensory, 305 Adenohypophysis Apoplexy, 196, 310 feedback system, 259 ARAS,295 hormone control, 257-259 Areflexia, 170 hypothalamus, 254 Arousal, 295 Adenosine triphosphate, 132-134, 147, 226 Arterial pressure, 247, s.a. -

Patient-Tailored Connectomics Visualization for the Assessment of White Matter Atrophy in Traumatic Brain Injury
Patient-Tailored Connectomics Visualization for the Assessment of White Matter Atrophy in Traumatic Brain Injury The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Irimia, Andrei, Micah C. Chambers, Carinna M. Torgerson, Maria Filippou, David A. Hovda, Jeffry R. Alger, Guido Gerig, et al. 2012. Patient-tailored connectomics visualization for the assessment of white matter atrophy in traumatic brain injury. Frontiers in Neurology 3:10. Published Version doi://10.3389/fneur.2012.00010 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:8462352 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA METHODS ARTICLE published: 06 February 2012 doi: 10.3389/fneur.2012.00010 Patient-tailored connectomics visualization for the assessment of white matter atrophy in traumatic brain injury Andrei Irimia1, Micah C. Chambers 1, Carinna M.Torgerson1, Maria Filippou 2, David A. Hovda2, Jeffry R. Alger 3, Guido Gerig 4, Arthur W.Toga1, Paul M. Vespa2, Ron Kikinis 5 and John D. Van Horn1* 1 Laboratory of Neuro Imaging, Department of Neurology, University of California Los Angeles, Los Angeles, CA, USA 2 Brain Injury Research Center, Departments of Neurology and Neurosurgery, University of California Los Angeles, Los Angeles, CA, USA 3 Department of Radiology, David -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -
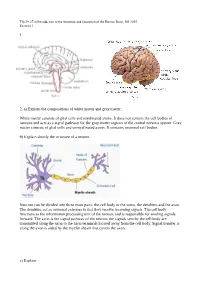
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter.