Michigan-Specific Reporting Requirements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
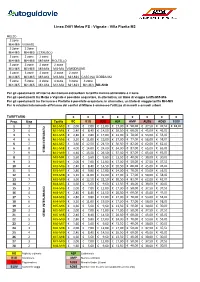
Polimetriche Per Linea STIBM Area Nord.Xlsx
Linea Z401 Melzo FS - Vignate - Villa Fiorita M2 MELZO 2 zone Mi4-Mi5 VIGNATE 2 zone 2 zone Mi4-Mi5 Mi4-Mi5 CERNUSCO 2 zone 2 zone 2 zone Mi4-Mi5 Mi4-Mi5 Mi3-Mi4 PIOLTELLO 3 zone 3 zone 2 zone 2 zone Mi3-Mi5 Mi3-Mi5 Mi3-Mi4 Mi3-Mi4 VIMODRONE 3 zone 3 zone 2 zone 2 zone 2 zone Mi3-Mi5 Mi3-Mi5 Mi3-Mi4 Mi3-Mi4 Mi3-Mi4 CASCINA GOBBA M2 5 zone 5 zone 4 zone 4 zone 3 zone 3 zone Mi1-Mi5 Mi1-Mi5 Mi1-Mi4 Mi1-Mi4 Mi1-Mi3 Mi1-Mi3 MILANO Per gli spostamenti all'interno dei Comuni extraurbani la tariffa minima utilizzabile è 2 zone Per gli spostamenti tra Melzo e Vignate è possibile acquistare, in alternativa, un titolo di viaggio tariffa Mi5-Mi6 Per gli spostamenti tra Cernusco e Pioltello è possibile acquistare, in alternativa, un titolo di viaggio tariffa Mi4-Mi5 Per le relazioni interamente all'interno dei confini di Milano è ammesso l'utilizzo di mensili e annuali urbani TARIFFARIO €€€€€€€€ Prog. Ring Tariffa BO B1G B3G ASP AMP AU26 AO65 B10V 1 3 Mi1-Mi3 € 2,00 € 7,00 € 12,00 € 17,00 € 50,00 € 37,50 € 37,50 € 18,00 2 4 Mi1-Mi4 € 2,40 € 8,40 € 14,50 € 20,50 € 60,00 € 45,00 € 45,00 3 5 Mi1-Mi5 € 2,80 € 9,80 € 17,00 € 24,00 € 70,00 € 53,00 € 53,00 4 6 Mi1-Mi6 € 3,20 € 11,00 € 19,00 € 27,00 € 77,00 € 58,00 € 58,00 5 7 Mi1-Mi7 € 3,60 € 12,50 € 21,50 € 30,50 € 82,00 € 62,00 € 62,00 6 8 Mi1-Mi8 € 4,00 € 14,00 € 24,00 € 34,00 € 87,00 € 65,00 € 65,00 7 9STIBM INTEGRATO Mi1-Mi9€ 4,40 € 15,50 € 26,50 € 37,50 € 87,00 € 65,00 € 65,00 8 2 MI3-MI4 € 1,60 € 5,60 € 9,60 € 13,50 € 40,00 € 30,00 € 30,00 9 3 MI3-MI5 € 2,00 € 7,00 € 12,00 € 17,00 € 50,00 € 37,50 -

A Visual Motion Detection Circuit Suggested by Drosophila Connectomics
ARTICLE doi:10.1038/nature12450 A visual motion detection circuit suggested by Drosophila connectomics Shin-ya Takemura1, Arjun Bharioke1, Zhiyuan Lu1,2, Aljoscha Nern1, Shiv Vitaladevuni1, Patricia K. Rivlin1, William T. Katz1, Donald J. Olbris1, Stephen M. Plaza1, Philip Winston1, Ting Zhao1, Jane Anne Horne2, Richard D. Fetter1, Satoko Takemura1, Katerina Blazek1, Lei-Ann Chang1, Omotara Ogundeyi1, Mathew A. Saunders1, Victor Shapiro1, Christopher Sigmund1, Gerald M. Rubin1, Louis K. Scheffer1, Ian A. Meinertzhagen1,2 & Dmitri B. Chklovskii1 Animal behaviour arises from computations in neuronal circuits, but our understanding of these computations has been frustrated by the lack of detailed synaptic connection maps, or connectomes. For example, despite intensive investigations over half a century, the neuronal implementation of local motion detection in the insect visual system remains elusive. Here we develop a semi-automated pipeline using electron microscopy to reconstruct a connectome, containing 379 neurons and 8,637 chemical synaptic contacts, within the Drosophila optic medulla. By matching reconstructed neurons to examples from light microscopy, we assigned neurons to cell types and assembled a connectome of the repeating module of the medulla. Within this module, we identified cell types constituting a motion detection circuit, and showed that the connections onto individual motion-sensitive neurons in this circuit were consistent with their direction selectivity. Our results identify cellular targets for future functional investigations, and demonstrate that connectomes can provide key insights into neuronal computations. Vision in insects has been subject to intense behavioural1,physiological2 neuroanatomy14. Given the time-consuming nature of such recon- and anatomical3 investigations, yet our understanding of its underlying structions, we wanted to determine the smallest medulla volume, neural computations is still far from complete. -

Notes and References Documents Held at the Public Record Office, London, Are Crown Copyright and Are Reproduced by Permission of the Controller Ofhm Stationery Office
Notes and References Documents held at the Public Record Office, London, are crown copyright and are reproduced by permission of the Controller ofHM Stationery Office. I NTRODUCTION Christopher Andrew and David Dilks I. David Dilks (ed.), The Diaries rifSir Alexander Cadogan O.M. 1938-1945 (Lon don , (971) , p. 21. 2. Interview with Professor Hinsley in Part 3 of the BBC Radio 4 documentary series 'T he Profession of Intelligence', written and presented by Christopher Andrew (producer Peter Everett); first broadcast 16 Aug 1981. 3. F. H. Hinsleyet al., British Intelligencein the Second World War (London, 1979-). The first two chapters of volume I contain a useful retrospect on the pre-war development of the intelligence community. Curiously, despite the publication of Professor Hinsley's volumes, the government has decided not to release the official histories commissioned by it on wartime counter-espionage and deception. The forthcoming (non-official) collection of essays edited by Ernest R. May, Knowing One's Enemies: IntelligenceAssessment before the Two World Wars (Princeton) promises to add significantly to our knowledge of the role of intelligence on the eve of the world wars. 4. House of Commons Education, Science and Arts Committee (Session 1982-83) , Public Records: Minutes ofEvidence, pp . 76-7. 5. Chapman Pincher, Their Trade is Treachery (London, 1981). Nigel West, A Matter of Trust: MI51945-72 (London, 1982). Both volumes contain ample evidence of extensive 'inside information'. 6. Nigel West , MI5: British Security Operations /90/-/945 (London, 1981), pp . 41, 49, 58. One of the most interesting studies of British peacetime intelligence which depends on a substantial amount of inside information is Antony Verrier's history of post-war British foreign policy , Through the Looking Glass (London, 1983) . -

UK Eyes Alpha by the Same Author UK Eyes Alpha Big Boys' Rules: the SAS and the Secret Struggle Against the IRA Lnside British Lntelligence
UK Eyes Alpha By the same author UK Eyes Alpha Big Boys' Rules: The SAS and the secret struggle against the IRA lnside British lntelligence Mark Urban tr firhrr anr/ fulrr' ft For Ruth and Edwin Contents lntroduction Part One The First published in I996 1 Coming Earthquake 3 and Faber Limited by Faber 2 A Dark and Curious Shadow 13 3 Queen Square London vcrN JAU 3 The Charm Offensive 26 Typeset by Faber and Faber Ltd Printed in England by Clays Ltd, St Ives plc 4 Most Ridiculed Service 42 All rights reserved 5 ZIRCON 56 O Mark Urban, 1996 6 Springtime for Sceptics 70 Mark Urbar-r is hereby identified as author of 7 A Brilliant Intelligence Operation 84 this work in accordance with Section 77 of the Copyright, Designs and Patents Act 1988 8 The \7all Comes Tumbling Down 101 A CIP rccord for this book is available from the Part Two British Library 9 Supergun LL7 tsnN o-57r-r7689-5 10 Black Death on the Nevsky Prospekt L29 ll Assault on Kuwait L43 12 Desert Shield 153 13 Desert Storm 165 14 Moscow Endgame LA2 Part Three l5 An Accidcnt of History L97 l(r Irrlo thc ll:rllirrn 2LO tt),)B / (,1,1 l, I Qulgrnirc 17 Time for Revenge 22L lntroduction 18 Intelligence, Power and Economic Hegemony 232 19 Very Huge Bills 245 How good is British intelligence? What kind of a return do ministers and officials get 20 The Axe Falls 2il for the hundreds of millions of pounds spent on espionage each year? How does this secret establishment find direction and purpose 2l Irish Intrigues 269 in an age when old certainties have evaporated? Very few people, even in Conclusion 286 Whitehall, would feel confident enough to answer these questions. -

Cambia Il Modo Di Viaggiare
Cambia il modo di viaggiare Nuovo sistema tariffario integrato dei mezzi pubblici www.trenord.it | App Trenord Indice 11 Il sistema tariffario integrato del bacino di mobilità (STIBM) 6 1.1 La suddivisione del territorio del bacino di mobilità in zone tariffarie 6 Tabella 1 Elenco in ordine alfabetico dei comuni ubicati nella zona tariffaria denominata Mi3 7 Tabella 2 Elenco in ordine alfabetico dei comuni ubicati nelle zone tariffarie Mi4, Mi5, Mi6, Mi7, Mi8, Mi9 8 Mappa del sistema tariffario integrato del bacino di mobilità (stibm) Milano - Monza Brianza 12 1.2 Il calcolo della tariffa 14 1.3 Le principali novità del nuovo sistema tariffario 15 22 Tipologie di spostamento e titoli di viaggio 16 2.1 Per spostarsi a Milano 16 2.2 Per spostarsi tra Milano e le località extraurbane 16 2.3 Per spostarsi tra località extraurbane senza attraversare i confini di Milano 17 2.4 Per spostarsi tra località extraurbane attraversando i confini di Milano 17 33 I nuovi titoli di viaggio 18 3.1 Titoli di viaggio ordinari 18 3.2 Titoli di viaggio agevolati e gratuità per ragazzi 19 3.3 Le informazioni presenti sul titolo di viaggio 20 3.4 Regole generali di utilizzo del titolo di viaggio 21 3.5 Il Biglietto Chip On Paper 22 44 Tariffe dei titoli di viaggio ordinari e dei titoli di viaggio agevolati 24 Tabella 3 Biglietti ordinari e altri titoli di viaggio occasionali 25 Tabella 4 Abbonamenti ordinari 26 Tabella 5 Abbonamenti agevolati 27 2 | IL NUOVO SISTEMA TARIFFARIO INTEGRATO - MILANO - MONZA BRIANZA 3 IL NUOVO SISTEMA Mi9 TARIFFARIO INTEGRATO MILANO -

Monday/Tuesday Playoff Schedule
2013 TUC MONDAY/TUESDAY PLAYOFF MASTER FIELD SCHEDULE Start End Hockey1 Hockey2 Hockey3 Hockey4 Hockey5 Ulti A Soccer 3A Soccer 3B Cricket E1 Cricket E2 Cricket N1 Cricket N2 Field X 8:00 9:15 MI13 MI14 TI13 TI14 TI15 TI16 MI1 MI2 MI3 MI4 MI15 MI16 9:20 10:35 MI17 MI18 TI17 TI18 TI19 TI20 MI5 MI6 10:40 11:55 MI19 MI20 MC1 MC2 MC3 MI21 MI7 MI8 12:00 1:15 MI9* TI21* TI22 TI23 TI24 MI10 MI11 MI12 1:20 2:35 MI22 MC4 MC6 MC5 MI23 TC1 MI24 MI25 2:40 3:55 TI1 TI2 MC7 TI3 MI26 TC2 TR1 TR2 MI27 4:00 5:15 MC8* TC3 MC10 MC9 TI4 TC4 TR3 TR4 5:20 6:35 TC5* TI5 TI6 TI7 TI8 TC6 TR5 TR6 6:40 7:55 TI9* TC7 TI10 TI11 TI12 TC8 TR8 TR7 Games are to 15 points Half time at 8 points Games are 1 hour and 15 minutes long Soft cap is 10 minutes before the end of game, +1 to highest score 2 Timeouts per team, per game NO TIMEOUTS AFTER SOFT CAP Footblocks not allowed, unless captains agree otherwise 2013 TUC Monday Competitive Playoffs - 1st to 7th Place 3rd Place Bracket Loser of MC4 Competitive Teams Winner of MC9 MC9 Allth Darth (1) Allth Darth (1) 3rd Place Slam Dunks (2) Loser of MC5 The Ligers (3) Winner of MC4 MC4 Krash Kart (4) Krash Kart (4) The El Guapo Sausage Party (5) MC1 Wonky Pooh (6) Winner of MC1 Disc Horde (7) The El Guapo Sausage Winner of MC8 Party (5) MC8 Slam Dunks (2) Champions Winner of MC2 MC2 Disc Horde (7) MC5 The Ligers (3) Winner of MC5 MC3 Winner of MC3 Wonky Pooh (6) Time Hockey3 Score Spirit Hockey4 Score Spirit Hockey5 Score Spirit Score Spirit 10:40 Krash Kart (4) Slam Dunks (2) The Ligers (3) to vs. -

Parus Caeruleus)
PATTERNS AND SIGNIFICANCE OF GEOGRAPHICAL VARIATION IN THE BLUE TIT (PARUS CAERULEUS) JEAN-LOUIS MARTIN CentreNational de la RechercheScientifique et Centre d'l•cologie Fontionnelle et Evolutive, (CEFE/CNRS), B.P. 5051, F-34033 MontpellierCedex, France, • and AmericanMuseum of Natural History,Department of Ornithology, Central Park West at 79th Street, New York, New York 10024-5192 USA ABSTRACT.--Istudied geographicalvariation in mensural charactersand plumage pattern over the breeding range of the Blue Tit (Paruscaeruleus). In Eurasiabody size decreases clinally from north-northeastto south-southwest,and bill thinnessand relative tarsallength increaseclinally from the northeastto the southwest.This variationis consistentwith changes in environmental factorsknown to affect these characters.Geographical isolates in North Africavary in sizeand in bill andtarsus morphology in a way consistentwith habitatvariation among isolates.Similarities in mensural charactersamong samplesare more consistentwith similaritiesin ecologicalconditions than with taxonomy.Geographical variation in plumage pattern essentiallyis clinal in Eurasiabut discontinuousin North Africa. On a broad scale, plumagepattern variation is consistentwith the populationgroups defined by taxonomists (Eurasianand North African populationgroups). Within eachpopulation group consistency between variation in plumage pattern and the probable population phylogeny is poor. My resultssubstantiate the importanceof the unifying effectof gene flow in adjacentsamples even over a large geographicalarea -

How Intelligence Agencies Are Adapting to Cyber
http://researchcommons.waikato.ac.nz/ Research Commons at the University of Waikato Copyright Statement: The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). The thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. Authors control the copyright of their thesis. You will recognise the author’s right to be identified as the author of the thesis, and due acknowledgement will be made to the author where appropriate. You will obtain the author’s permission before publishing any material from the thesis. Intelligence Agencies in Cyberspace: Adapting the Intelligence Cycle to Cyber Threats and Opportunities A thesis submitted in partial fulfilment of the requirements for the degree of Master of Social Sciences at The University of Waikato by Jedediah Warwick Greenwood 2020 1 Abstract Intelligence has grown and changed dramatically over the past hundred years with the advent of cyberspace. This thesis will begin by examining how the intelligence cycle has adapted to accommodate cyber threats and opportunities, before conducting three national case studies examining the organisational changes in the signals intelligence agencies in New Zealand, the United Kingdom, and the United States of America. It will utilise the analysis of how the intelligence cycle and States have grown to accommodate cyber phenomenon and will conduct two case studies on the recent events concerning Huawei and the hacking of the 2016 US Election. -

Michigan-Specific Reporting Update Memorandum
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE-MEDICAID COORDINATION OFFICE DATE: February 28, 2020 TO: Medicare-Medicaid Plans in Michigan FROM: Lindsay P. Barnette Director, Models, Demonstrations and Analysis Group SUBJECT: Revised Michigan-Specific Reporting Requirements and Value Sets Workbook The purpose of this memorandum is to announce the release of the revised Medicare-Medicaid Capitated Financial Alignment Model Reporting Requirements: Michigan-Specific Reporting Requirements and corresponding Michigan-Specific Value Sets Workbook. These documents provide updated guidance, technical specifications, and applicable codes for the state-specific measures that Michigan Medicare-Medicaid Plans (MMPs) are required to collect and report under the demonstration. As with prior annual update cycles, revisions were made in an effort to streamline and clarify reporting expectations for Michigan MMPs. Please see below for a summary of the substantive changes to the Michigan-Specific Reporting Requirements. Note that the Michigan-Specific Value Sets Workbook also includes changes; Michigan MMPs should carefully review and incorporate the updated value sets, particularly for measures MI2.5, MI5.6, MI7.1, and MI7.3. Michigan MMPs must use the updated specifications and value sets for measures due on or after June 1, 2020. Michigan MMPs must also reference the latest Prevention Quality Indicators (PQI) technical specifications when reporting measure MI5.1 on April 30, 2020. Should you have any questions, please contact the Medicare-Medicaid Coordination Office at [email protected]. SUMMARY OF CHANGES Introduction • In the “Variations from the Core Reporting Requirements Document” section, updated the Michigan-specific guidance regarding data sources for reporting Core Measure 9.2. -

DEFENCE STRATEGIC COMMUNICATIONS the Official Journal of the NATO Strategic Communications Centre of Excellence
ISSN 2500-9478 Volume 1 | Number 1 | Winter 2015 DEFENCE STRATEGIC COMMUNICATIONS The official journal of the NATO Strategic Communications Centre of Excellence Russia’s 21st century information war. Moving past the ‘Funnel’ Model of Counterterrorism Communication. Assessing a century of British military Information Operations. Memetic warfare. The constitutive narratives of Daesh. Method for minimizing the negative consequences of nth order effects in StratCom. The Narrative and Social Media. Public Diplomacy and NATO. 2 ISSN 2500-9478 Defence Strategic Communications Editor-in-Chief Dr. Steve Tatham Editor Anna Reynolds Production and Copy Editor Linda Curika Editorial Board Matt Armstrong, MA Dr. Emma Louise Briant Dr. Nerijus Maliukevicius Thomas Elkjer Nissen, MA Dr. Žaneta Ozolina Dr. Agu Uudelepp Dr. J. Michael Waller Dr. Natascha Zowislo-Grünewald “Defence Strategic Communications” is an international peer-reviewed journal. The journal is a project of the NATO Strategic Communications Centre of Excellence (NATO StratCom COE). It is produced for NATO, NATO member countries, NATO partners, related private and public institutions, and related individuals. It does not represent the opinions or policies of NATO or NATO StratCom COE. The views presented in the following articles are those of the authors alone. © All rights reserved by the NATO StratCom COE. Articles may not be copied, reproduced, distributed or publicly displayed without reference to the NATO StratCom COE and the academic journal. NATO Strategic Communications Centre of Excellence Riga, Kalnciema iela 11b, Latvia LV1048 www.stratcomcoe.org Ph.: 0037167335463 [email protected] 3 INTRODUCTION I am delighted to welcome you to the first edition of ‘Defence Strategic Communications’ Journal. -
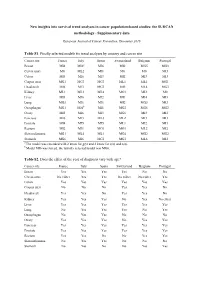
New Insights Into Survival Trend Analyses in Cancer Population-Based Studies: the SUDCAN Methodology - Supplementary Data
New insights into survival trend analyses in cancer population-based studies: the SUDCAN methodology - Supplementary data European Journal of Cancer Prevention, December 2016 Table S1. Finally selected models for trend analyses by country and cancer site Cancer site France Italy Spain Switzerland Belgium Portugal Breast MI4 MI6 a MI6 MI2 MG5 MG3 Cervix uteri M0 ML2 MI1 M0 M0 MI3 Colon MI3 MI6 MI3 MI2 MI3 MI3 Corpus uteri MG1 MG3 MG3 ML1 ML1 MG1 Head-neck MI6 MI3 MG3 MI5 ML4 MG3 Kidney ML1 ML3 ML4 MG1 MI2 M0 Liver MI3 MI6 MI2 MI1 MI4 MI3 Lung MG3 MI6 MI6 MI2 MG3 MI3 Oesophagus MG1 MI6 b MI1 MG2 MG6 MG2 Ovary MI5 MI6 MI3 MG6 MI3 MI2 Pancreas MI6 MI3 ML3 ML2 MI3 MI3 Prostate MI4 MI5 MI5 ML1 MI2 MI3 Rectum MI2 MI1 MG1 MG1 ML2 MI2 Skin melanoma MG1 ML4 ML1 MG4 MG2 MG2 Stomach MG6 MI6 MG1 MG1 ML6 MI2 a The model was extended with 2 knots for g(y) and 2 knots for s(a) and n(a) . b Model MI6 was forced, the initially selected model was MG6. Table S2. Does the effect of the year of diagnosis vary with age? Cancer site France Italy Spain Switzerland Belgium Portugal Breast Yes Yes Yes Yes No No Cervix uteri No effect Yes Yes No effect No effect Yes Colon Yes Yes Yes Yes Yes Yes Corpus uteri No No No Yes Yes No Head-neck Yes Yes No Yes Yes No Kidney Yes Yes Yes No Yes No effect Liver Yes Yes Yes Yes Yes Yes Lung No Yes Yes Yes No Yes Oesophagus No Yes Yes No No No Ovary Yes Yes Yes No Yes Yes Pancreas Yes Yes Yes Yes Yes Yes Prostate Yes Yes Yes Yes Yes Yes Rectum Yes Yes No No Yes Yes Skin melanoma No Yes Yes No No No Stomach No Yes No No Yes Yes Table S3. -

The British Intelligence Community in Singapore, 1946-1959: Local
The British intelligence community in Singapore, 1946-1959: Local security, regional coordination and the Cold War in the Far East Alexander Nicholas Shaw Submitted in accordance with the requirements for the degree of PhD The University of Leeds, School of History January 2019 The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Alexander Nicholas Shaw to be identified as Author of this work has been asserted by Alexander Nicholas Shaw in accordance with the Copyright, Designs and Patents Act 1988. Acknowledgements I would like to thank all those who have supported me during this project. Firstly, to my funders, the White Rose College of the Arts and Humanities and the Arts and Humanities Research Council. Caryn Douglas and Clare Meadley have always been most encouraging and have never stinted in supplying sausage rolls. At Leeds, I am grateful to my supervisors Simon Ball, Adam Cathcart and, prior to his retirement, Martin Thornton. Emma Chippendale and Joanna Phillips have been invaluable guides in navigating the waters of PhD admin. In Durham, I am indebted to Francis Gotto from Palace Green Library and the Oriental Museum’s Craig Barclay and Rachel Barclay. I never expected to end up curating an exhibition of Asian art when I started researching British intelligence, but Rachel and Craig made that happen.