Parus Caeruleus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Michigan-Specific Reporting Requirements
Version: February 28, 2020 MEDICARE-MEDICAID CAPITATED FINANCIAL ALIGNMENT MODEL REPORTING REQUIREMENTS: MICHIGAN-SPECIFIC REPORTING REQUIREMENTS Issued February 28, 2020 MI-1 Version: February 28, 2020 Table of Contents Michigan-Specific Reporting Requirements Appendix ......................................... MI-3 Introduction ....................................................................................................... MI-3 Definitions .......................................................................................................... MI-3 Variations from the Core Reporting Requirements Document ..................... MI-4 Quality Withhold Measures .............................................................................. MI-5 Reporting on Assessments and IICSPs Completed Prior to First Effective Enrollment Date ................................................................................. MI-6 Guidance on Assessments and IICSPs for Members with a Break in Coverage ............................................................................................................ MI-6 Reporting on Passively Enrolled and Opt-In Enrolled Members .................. MI-8 Reporting on Disenrolled and Retro-disenrolled Members ........................... MI-9 Hybrid Sampling ................................................................................................ MI-9 Value Sets ........................................................................................................ MI-10 Michigan’s Implementation, Ongoing, -

Monday/Tuesday Playoff Schedule
2013 TUC MONDAY/TUESDAY PLAYOFF MASTER FIELD SCHEDULE Start End Hockey1 Hockey2 Hockey3 Hockey4 Hockey5 Ulti A Soccer 3A Soccer 3B Cricket E1 Cricket E2 Cricket N1 Cricket N2 Field X 8:00 9:15 MI13 MI14 TI13 TI14 TI15 TI16 MI1 MI2 MI3 MI4 MI15 MI16 9:20 10:35 MI17 MI18 TI17 TI18 TI19 TI20 MI5 MI6 10:40 11:55 MI19 MI20 MC1 MC2 MC3 MI21 MI7 MI8 12:00 1:15 MI9* TI21* TI22 TI23 TI24 MI10 MI11 MI12 1:20 2:35 MI22 MC4 MC6 MC5 MI23 TC1 MI24 MI25 2:40 3:55 TI1 TI2 MC7 TI3 MI26 TC2 TR1 TR2 MI27 4:00 5:15 MC8* TC3 MC10 MC9 TI4 TC4 TR3 TR4 5:20 6:35 TC5* TI5 TI6 TI7 TI8 TC6 TR5 TR6 6:40 7:55 TI9* TC7 TI10 TI11 TI12 TC8 TR8 TR7 Games are to 15 points Half time at 8 points Games are 1 hour and 15 minutes long Soft cap is 10 minutes before the end of game, +1 to highest score 2 Timeouts per team, per game NO TIMEOUTS AFTER SOFT CAP Footblocks not allowed, unless captains agree otherwise 2013 TUC Monday Competitive Playoffs - 1st to 7th Place 3rd Place Bracket Loser of MC4 Competitive Teams Winner of MC9 MC9 Allth Darth (1) Allth Darth (1) 3rd Place Slam Dunks (2) Loser of MC5 The Ligers (3) Winner of MC4 MC4 Krash Kart (4) Krash Kart (4) The El Guapo Sausage Party (5) MC1 Wonky Pooh (6) Winner of MC1 Disc Horde (7) The El Guapo Sausage Winner of MC8 Party (5) MC8 Slam Dunks (2) Champions Winner of MC2 MC2 Disc Horde (7) MC5 The Ligers (3) Winner of MC5 MC3 Winner of MC3 Wonky Pooh (6) Time Hockey3 Score Spirit Hockey4 Score Spirit Hockey5 Score Spirit Score Spirit 10:40 Krash Kart (4) Slam Dunks (2) The Ligers (3) to vs. -

Michigan-Specific Reporting Update Memorandum
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE-MEDICAID COORDINATION OFFICE DATE: February 28, 2020 TO: Medicare-Medicaid Plans in Michigan FROM: Lindsay P. Barnette Director, Models, Demonstrations and Analysis Group SUBJECT: Revised Michigan-Specific Reporting Requirements and Value Sets Workbook The purpose of this memorandum is to announce the release of the revised Medicare-Medicaid Capitated Financial Alignment Model Reporting Requirements: Michigan-Specific Reporting Requirements and corresponding Michigan-Specific Value Sets Workbook. These documents provide updated guidance, technical specifications, and applicable codes for the state-specific measures that Michigan Medicare-Medicaid Plans (MMPs) are required to collect and report under the demonstration. As with prior annual update cycles, revisions were made in an effort to streamline and clarify reporting expectations for Michigan MMPs. Please see below for a summary of the substantive changes to the Michigan-Specific Reporting Requirements. Note that the Michigan-Specific Value Sets Workbook also includes changes; Michigan MMPs should carefully review and incorporate the updated value sets, particularly for measures MI2.5, MI5.6, MI7.1, and MI7.3. Michigan MMPs must use the updated specifications and value sets for measures due on or after June 1, 2020. Michigan MMPs must also reference the latest Prevention Quality Indicators (PQI) technical specifications when reporting measure MI5.1 on April 30, 2020. Should you have any questions, please contact the Medicare-Medicaid Coordination Office at [email protected]. SUMMARY OF CHANGES Introduction • In the “Variations from the Core Reporting Requirements Document” section, updated the Michigan-specific guidance regarding data sources for reporting Core Measure 9.2. -
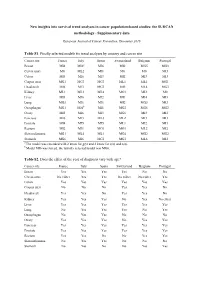
New Insights Into Survival Trend Analyses in Cancer Population-Based Studies: the SUDCAN Methodology - Supplementary Data
New insights into survival trend analyses in cancer population-based studies: the SUDCAN methodology - Supplementary data European Journal of Cancer Prevention, December 2016 Table S1. Finally selected models for trend analyses by country and cancer site Cancer site France Italy Spain Switzerland Belgium Portugal Breast MI4 MI6 a MI6 MI2 MG5 MG3 Cervix uteri M0 ML2 MI1 M0 M0 MI3 Colon MI3 MI6 MI3 MI2 MI3 MI3 Corpus uteri MG1 MG3 MG3 ML1 ML1 MG1 Head-neck MI6 MI3 MG3 MI5 ML4 MG3 Kidney ML1 ML3 ML4 MG1 MI2 M0 Liver MI3 MI6 MI2 MI1 MI4 MI3 Lung MG3 MI6 MI6 MI2 MG3 MI3 Oesophagus MG1 MI6 b MI1 MG2 MG6 MG2 Ovary MI5 MI6 MI3 MG6 MI3 MI2 Pancreas MI6 MI3 ML3 ML2 MI3 MI3 Prostate MI4 MI5 MI5 ML1 MI2 MI3 Rectum MI2 MI1 MG1 MG1 ML2 MI2 Skin melanoma MG1 ML4 ML1 MG4 MG2 MG2 Stomach MG6 MI6 MG1 MG1 ML6 MI2 a The model was extended with 2 knots for g(y) and 2 knots for s(a) and n(a) . b Model MI6 was forced, the initially selected model was MG6. Table S2. Does the effect of the year of diagnosis vary with age? Cancer site France Italy Spain Switzerland Belgium Portugal Breast Yes Yes Yes Yes No No Cervix uteri No effect Yes Yes No effect No effect Yes Colon Yes Yes Yes Yes Yes Yes Corpus uteri No No No Yes Yes No Head-neck Yes Yes No Yes Yes No Kidney Yes Yes Yes No Yes No effect Liver Yes Yes Yes Yes Yes Yes Lung No Yes Yes Yes No Yes Oesophagus No Yes Yes No No No Ovary Yes Yes Yes No Yes Yes Pancreas Yes Yes Yes Yes Yes Yes Prostate Yes Yes Yes Yes Yes Yes Rectum Yes Yes No No Yes Yes Skin melanoma No Yes Yes No No No Stomach No Yes No No Yes Yes Table S3. -

The British Intelligence Community in Singapore, 1946-1959: Local
The British intelligence community in Singapore, 1946-1959: Local security, regional coordination and the Cold War in the Far East Alexander Nicholas Shaw Submitted in accordance with the requirements for the degree of PhD The University of Leeds, School of History January 2019 The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Alexander Nicholas Shaw to be identified as Author of this work has been asserted by Alexander Nicholas Shaw in accordance with the Copyright, Designs and Patents Act 1988. Acknowledgements I would like to thank all those who have supported me during this project. Firstly, to my funders, the White Rose College of the Arts and Humanities and the Arts and Humanities Research Council. Caryn Douglas and Clare Meadley have always been most encouraging and have never stinted in supplying sausage rolls. At Leeds, I am grateful to my supervisors Simon Ball, Adam Cathcart and, prior to his retirement, Martin Thornton. Emma Chippendale and Joanna Phillips have been invaluable guides in navigating the waters of PhD admin. In Durham, I am indebted to Francis Gotto from Palace Green Library and the Oriental Museum’s Craig Barclay and Rachel Barclay. I never expected to end up curating an exhibition of Asian art when I started researching British intelligence, but Rachel and Craig made that happen. -

2014 Tuc Monday/Tuesday Playoff Master Field Schedule
2014 TUC MONDAY/TUESDAY PLAYOFF MASTER FIELD SCHEDULE Start End Hockey 1 Hockey 2 Hockey 3 Hockey 4 Hockey 5 Hockey 6 Ulti A Rugby E1 Rugby E2 9:20 10:35 MI1 MI2 MC1 MC2 MI8 MI9 10:40 11:55 MI3 MI4 MC5 MC3 MC4 MI11 MI5 MI10 12:00 1:15 MI6 MI7 MC7 1:30 2:55 ASI1 ASI2 ASC1 ASC2 MC6 3:00 4:25 ASI3 ASI4 ASC4 ASC3 4:30 5:45 T5 T6 T2 T1 5:50 7:05 T7 T8 T4 T3 TOURNAMENT RULES Games are to 15 points Half time at 8 points Playoff games are 1 hour and 15 minutes long Hard cap is at 75 minutes (finish the point, only if tied do you play another), and there is no soft cap. 1 Timeout per half, per team (no timeouts if in overtime) Footblocks are not allowed, unless captains agree otherwise 2014 TUC Monday Competitive Playoffs - 1st to 6th Place Teams 3rd Place Deep (1) Deep (1) L - MC3 Allth Darth (2) 3rd Place MC7 naptime (3) W - MC3 MC3 Slam Dunks (4) Slam Dunks (4) L - MC4 El Guapo (5) MC1 Basic Bishes (6) W - MC1 5th Place El Guapo (5) Champions L - MC1 MC6 5th Place MC5 Allth Darth (2) L - MC2 MC4 naptime (3) W - MC4 MC2 W - MC2 Basic Bishes (6) Time Hockey 4 Score Spirit Hockey 6 Score Spirit 9:20 Slam Dunks (4) naptime (3) to vs. MC 1 vs. MC 2 10:35 El Guapo (5) Basic Bishes (6) Time Hockey 4 Score Spirit Hockey 6 Score Spirit Hockey 3 Score Spirit 10:40 Deep (1) Allth Darth (2) L - MC1 to vs. -

British Intelligence Analysis and the German Army at the Battle of Broodseinde, 4 October 1917
School of History and Cultures A Planned Massacre? British Intelligence Analysis and the German Army at the Battle of Broodseinde, 4 October 1917 By John Freeman Thesis submitted to The University of Birmingham In partial fulfilment for the degree of M Phil in Twentieth-Century British History School of History and Cultures July 2011 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Contents Acknowledgements Introduction p. 1 Chapter I. The Build-Up to the Battle of Broodseinde, 4 October 1917. p. 6 Introduction p. 7 The War Office p. 8 GHQ p. 14 The Armies p. 20 The Corps p. 26 The Divisions p. 31 The Intelligence Corps p. 35 Conclusion p. 41 Chapter II. Broodseinde: the Day by Day Intelligence Picture. p. 43 Introduction p. 44 28 September 1917 p. 45 29 September 1917 p. 49 30 September 1917 p. 52 1 October 1917 p. 57 2 October 1917 p. 62 3 October 1917 p. 67 Conclusion p. 71 Bibliography p. 74 Acknowledgements This study would not have been possible without the help of Gary Sheffield, Michael LoCicero and John Bourne. -

Michigan-Specific Reporting Update Memorandum
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE-MEDICAID COORDINATION OFFICE DATE: February 28, 2018 TO: Medicare-Medicaid Plans in Michigan FROM: Lindsay P. Barnette Director, Models, Demonstrations and Analysis Group SUBJECT: Revised Michigan-Specific Reporting Requirements and Value Sets Workbook The purpose of this memorandum is to announce the release of the revised Medicare-Medicaid Capitated Financial Alignment Model Reporting Requirements: Michigan-Specific Reporting Requirements and corresponding Michigan-Specific Value Sets Workbook. These documents provide updated guidance, technical specifications, and applicable codes for the state-specific measures that Michigan Medicare-Medicaid Plans (MMPs) are required to collect and report under the demonstration. Many of the updates were made in an effort to clarify and simplify reporting expectations for Michigan MMPs, consistent with the Administration’s commitment to reduce administrative burden and increase efficiency. Please see below for a summary of the substantive changes to the Michigan-Specific Reporting Requirements. Note that the Michigan-Specific Value Sets Workbook also includes changes; Michigan MMPs should carefully review and incorporate the updated value sets, particularly for measures MI2.5, MI2.6, MI5.3, MI5.6, and MI7.3. Michigan MMPs must use the updated specifications and value sets for measures due on or after May 31, 2018. Should you have any questions, please contact the Medicare-Medicaid Coordination Office at [email protected]. SUMMARY OF CHANGES Introduction • In the “Variations from the Core Document” section, added additional Michigan-specific guidance for reporting the new Core Measure 3.2 and updated the Michigan-specific guidance for reporting Core Measure 9.2. -

Intelligence and the War Against Japan: Britain, America and the Politics of Secret Service Richard J
Cambridge University Press 978-0-521-06619-8 - Intelligence and the War against Japan: Britain, America and the Politics of Secret Service Richard J. Aldrich Index More information Index Abbotabad, 251 Anglo-Japanese intelligence Abwehr co-operation, 106 decrypts, 143, 215, 242 Anglo-Thai Treaty, 324 in Britain, 49 Annam, 345 Acheson, Dean, 136, 323 Anson, Wilfred, 129 Adams, Alec, 195, 320–1 Anstey, Brigadier Sir John, 316 Adul, Luang, 195 Anti-Fascist Organisations (AFOs), 201, aerial reconnaissance, 50 336 Afghanistan, 151, 157, 165, 217, 265 Anti-Japanese United Front (AJUF), agent recruitment, 184–5, 219–20 190, 229, 330–4 Agnew, Lt Colonel, 367 archives, 214, 385 Air and Ground Forces Resources and Arlington Hall, 235, 248 Technical Staff (AGFRTS), 273 Armstrong Vickers Ltd, 31 Air Ground Aid Service (AGAS), 273, Asiatic Petroleum Ltd, 31 343, 360 Assam, 101 Atlantic Charter, 111 air intelligence, weakness of, 23, 31, 36 atomic bomb, xv, 307, 318 Airy, Lt Colonel E. A., 192 atomic intelligence, 317 Allied Forces headquarters (AFHQ), attack indicators, 49–51, 65–7 141 Attlee, Clement, 121, 178, 323–4 Allied Land Forces Para-Military Aung Sang, General, 199, 335 Operations (ALFPMO), 316, Australia, 89, 193 328–9, 352–3, 355 and ‘American imperialism’, 380–1 Amanullah, King of Afghanistan, 165 Australian Security Intelligence American–British–Dutch–Australian Organisation, 70 Command (ABDA), 156–7, 236, Axis intelligence co-operation, 46–7 241 Axis Planning Section, 53, 90 Amery, Julian, 288 Axis, surrender, xiv Amery, Leo, 158, 172, -

2021 Game Schedule
Mooresville Baseball League - 2021 Game Schedule Baseball MINOR ROOKIE PEEWEE INSTRUCTIONAL MAJOR Softball STB 7-8U (CP) 9-10U (KP) 11-12U (S) Opening Day - April 17 May 17 - May 22 Diamond Diamond Date Day Time Date Day Time 1 2 3 4 5 6 1 2 3 4 5 6 9:00 IN1-IN2 IN3-IN8 MA1-MA2 MA3-MA5 IN4-IN7 17-May Mon 6:00 S1-S2 IN2-IN7 MA5-MA1 MA2-MA4 MI2 - MI5 11:00 MI2 - MI1 MI3 - MI8 MI4 - MI7 MI5 - MI6 STB1-STB2 18-May Tue 6:00 MI2 - MI1 MI3 - MI8 MI4 - MI7 MI5 - MI6 17-Apr Sat 1:00 PW1-PW2 PW3-PW10 PW4-PW9 PW5-PW8 PW6-PW7 19-May Wed 6:00 S2-S3 IN3-IN6 MA2-MA5 MA4-MA3 PW2-PW9 3:00 R1-R4 R2-R3 CP1-CP2 CP3-CP4 KP1-KP2 20-May Thu 6:00 MI3 - MI4 MI1 - MI7 MI8 - MI6 MI2 - MI5 5:00 KP3-KP4 IN5-IN6 STB3-STB4 STB5-STB6 21-May Fri 6:00 CP2-CP3 CP4-CP1 KP1-KP2 KP3-KP4 9:00 IN4-IN5 IN8-IN1 STB3-STB6 STB4-STB1 April 19 - April 24 11:00 R3-R1 R4-R2 STB2-STB5 Diamond 22-May Sat 1:00 PW3-PW8 PW1-PW10 PW5-PW6 PW4-PW7 Date Day Time 1 2 3 4 5 6 3:00 19-Apr Mon 6:00 S1-S2 R1-R4 MA3-MA2 MA4-MA1 5:00 20-Apr Tue 6:00 Cancel Cancel Cancel Cancel 21-Apr Wed 6:00 Cancel Cancel Cancel Cancel May 24 - May 29 22-Apr Thu 6:00 S2-S3 R2-R3 MA1-MA3 MA5-MA4 IN8-IN6 Diamond Date Day Time 23-Apr Fri 6:00 PW2-PW3 PW1-PW7 PW8-PW6 PW9-PW5 PW10-PW4 1 2 3 4 5 6 9:00 IN3-IN4 IN2-IN5 STB1-STB6 STB2-STB4 IN1-IN7 24-May Mon 6:00 S3-S1 KP2-KP3 MA1-MA2 MA3-MA5 STB2-STB4 11:00 R1-R2 R3-R4 STB3-STB5 PW7-PW8 PW5-PW10 25-May Tue 6:00 MI6 - MI2 MI7 - MI8 MI4 - MI1 MI5 - MI3 STB1-STB6 24-Apr Sat 1:00 CP3-CP1 CP2-CP4 PW3-PW1 PW4-PW2 26-May Wed 6:00 S1-S2 KP4-KP1 MA3-MA2 MA4-MA1 3:00 27-May Thu 6:00 -

University of Groningen Design and Verification of Lock-Free Parallel
University of Groningen Design and verification of lock-free parallel algorithms Gao, Hui IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2005 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Gao, H. (2005). Design and verification of lock-free parallel algorithms. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 27-09-2021 Appendix A For lock-free dynamic hash tables A.1 Invariants Some abbreviations. Find(r, a) , r = null ∨ a = ADR(r) LeastFind(a, n) , (∀m<n : ¬Find(Y[key(a, curSize, m)], a)) ∧ Find(Y[key(a, curSize, n)], a)) LeastFind(h, a, n) , (∀m<n : ¬Find(h.table[key(a, h.size, m)], a)) ∧ Find(h.table[key(a, h.size, n)], a)) Axioms on functions key and ADR. -
Investigating the Diversity of the Terrestrial Invertebrate Fauna of Antarctica: a Closer Look at the Stereotydeus (Acari: Prostigmata) Genus
Investigating the diversity of the terrestrial invertebrate fauna of Antarctica: a closer look at the Stereotydeus (Acari: Prostigmata) genus Claudia Brunetti 1,*, Henk Siepel 2, Pietro Paolo Fanciulli 1, Francesco Nardi 1 and Antonio Carapelli 1 1 Department of Life Sciences, University of Siena, Via Aldo Moro 2, 53100 SI Siena, Italy; 2 Department of Animal Ecology and Physiology, Institute for Water and Wetland Research, Radboud University, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands * Corresponding author: [email protected] 1 Abstract: In the extreme Antarctic ecosystems, plants and invertebrates are confine to sparse and isolated ice-free refugia where they survived for millions of years and where they undergo differentiation and population divergence, potentially resulting in speciation. In invertebrates' populations, the gene flow is strongly affected and reduce by their limited dispersal abilities, their specific habitat requirements and the substantial geographical barriers. And thus, results in high genetic differentiation between clusters of individuals. Mites are surely the predominant invertebrate group of Continental Antarctica and the free-living genus Stereotydeus Berlese, 1901 (Acari: Prostigmata) is one of the most abundant along the coastal zones of Victoria Land and the Transantarctic Mountains. To examine the biodiversity and the phylogeographic distribution ranges of Stereotydeus spp. across Victoria Land, we conducted an integrated analysis of the genus through morphological, phylogenetic and population genetics studies. The results suggest a possible common evolutionary history in several isolated glacial refugia, with scarce gene flow even within populations probably resulting from inter/intra-specific events influenced by several abiotic/biotic factors. Keywords: Victoria Land; molecular phylogeny; biogeography; Acari; Stereotydeus spp.