The Cingulate Gyrus : Additional Motor Area and Cortical Autonomic Regulator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Stroke Movement Disorders: Report of 56 Patients F Alarco´N, J C M Zijlmans, G Duen˜As, N Cevallos
1568 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.011874 on 15 October 2004. Downloaded from PAPER Post-stroke movement disorders: report of 56 patients F Alarco´n, J C M Zijlmans, G Duen˜as, N Cevallos ............................................................................................................................... J Neurol Neurosurg Psychiatry 2004;75:1568–1574. doi: 10.1136/jnnp.2003.011874 Background: Although movement disorders that occur following a stroke have long been recognised in short series of patients, their frequency and clinical and imaging features have not been reported in large series of patients with stroke. Methods: We reviewed consecutive patients with involuntary abnormal movements (IAMs) following a stroke who were included in the Eugenio Espejo Hospital Stroke Registry and they were followed up for at least one year after the onset of the IAM. We determined the clinical features, topographical correlations, See end of article for authors’ affiliations and pathophysiological implications of the IAMs. ....................... Results: Of 1500 patients with stroke 56 developed movement disorders up to one year after the stroke. Patients with chorea were older and the patients with dystonia were younger than the patients with other Correspondence to: Dr. F Alarco´n, Department IAMs. In patients with isolated vascular lesions without IAMs, surface lesions prevailed but patients with of Neurology, Eugenio deep vascular lesions showed a higher probability of developing abnormal movements. One year after Espejo Hospital, P.O. Box onset of the IAMs, 12 patients (21.4%) completely improved their abnormal movements, 38 patients 17-07-9515, Quito, Ecuador, South America; (67.8%) partially improved, four did not improve (7.1%), and two patients with chorea died. -

Magnetic Resonance Imaging of Multiple Sclerosis: a Study of Pulse-Technique Efficacy
691 Magnetic Resonance Imaging of Multiple Sclerosis: A Study of Pulse-Technique Efficacy Val M. Runge1 Forty-two patients with the clinical diagnosis of multiple sclerosis were examined by Ann C. Price1 proton magnetic resonance imaging (MRI) at 0.5 T. An extensive protocol was used to Howard S. Kirshner2 facilitate a comparison of the efficacy of different pulse techniques. Results were also Joseph H. Allen 1 compared in 39 cases with high-resolution x-ray computed tomography (CT). MRI revealed characteristic abnormalities in each case, whereas CT was positive in only 15 C. Leon Partain 1 of 33 patients. Milder grades 1 and 2 disease were usually undetected by CT, and in all A. Everette James, Jr.1 cases, the abnormalities noted on MRI were much more extensive than on CT. Cerebral abnormalities were best shown with the T2-weighted spin-echo sequence (TE/TR = 120/1000); brainstem lesions were best defined on the inversion-recovery sequence (TE/TI/TR =30/400/1250). Increasing TE to 120 msec and TR to 2000 msec heightened the contrast between normal and abnormal white matter. However, the signal intensity of cerebrospinal fluid with this pulse technique obscured some abnormalities. The diagnosis of multiple sclerosis continues to be a clinical challenge [1,2). The lack of an objective means of assessment further complicates the evaluation of treatment regimens. Evoked potentials, cerebrospinal fluid (CSF) analysis , and computed tomography (CT) are currently used for diagnosis, but all lack sensitivity and/or specificity. Furthermore, postmortem examinations demonstrate many more lesions than those suggested by clinical means [3). -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
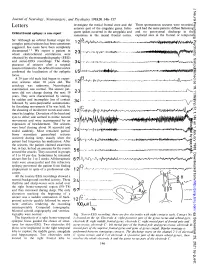
Letters Anterior Part of the Cingulate Gyrus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.1.146 on 1 January 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:146-157 investigate the mesial frontal zone and the Three spontaneous seizures were recorded, Letters anterior part of the cingulate gyrus. Infre- each had the same pattern: diffuse flattening quent spikes occurred in the amygdala and and no in the Orbital frontal epilepsy: a case report paroxysmal discharge sometimes in the mesial frontal cortex. explored sites in the frontal or temporal Sir: Although an orbital frontal origin for A complex partial seizures has been sometimes 12 suggested, few cases have been completely documented.1 2 We report a patient in 23 whom electroclinical correlations were obtained by electroencephalography (EEG) and stereo-EEG recordings. The disap- 3.4 I-w VI,-#a+r - pearance of seizures after a surgical resection limited to the orbital frontal cortex confirmed the localisation of the epileptic 4.5 o I\A-.ViJV "fJ4 WOO focus. A 29 year old male had begun to experi- B ence seizures when 10 years old. The aetiology was unknown. Neurological examination was normal. The seizure pat- terns did not change during the next 19 23 ., years. They were characterised by staring, r by sudden and incomplete loss of contact -- - -. , A- followed by semi-purposeful automatisms, 3-4 v by thrashing movements if he was held, by the shouting of incoherent words and some- times by laughter. Deviation of the head and eyes to either side seemed to mimic natural Protected by copyright. -

University of Florida Thesis Or Dissertation Formatting
THE NEURAL CIRCUITRY OF RESTRICTED REPETITIVE BEHAVIOR By BRADLEY JAMES WILKES A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Bradley James Wilkes To my father, Wade Wilkes, for his lifelong support, love, and encouragement ACKNOWLEDGMENTS This research was supported by funding from the Dissertation Research Award from the American Psychological Assocation, the Pilot Project Award (Non-Patient Oriented Clinical/Translational Research) from the Clinical and Translational Science Institute at the University of Florida, the Robert A. and Phyllis Levitt Award, the Gerber Behavioral and Cognitive Neuroscience Psychology Research Award, and the Jacquelin Goldman Scholarship in Developmental Psychology. I would especially like to thank Drs. Mark Lewis, Marcelo Febo, David Vaillancourt, Luis Colon-Perez, Darragh Devine, Timothy Vollmer, and Michael King for their support and guidance. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS .................................................................................................. 4 LIST OF TABLES ............................................................................................................ 7 LIST OF FIGURES .......................................................................................................... 8 LIST OF ABBREVIATIONS ........................................................................................... 10 ABSTRACT .................................................................................................................. -

Anatomic Significance of Topographical Relief in the Pars Basalis Telencephali
Rom J Leg Med [27] 1-9 [2019] DOI: 10.4323/rjlm.2019.1 © 2019 Romanian Society of Legal Medicine FUNDAMENTAL RESEARCH Anatomic significance of topographical relief in the pars basalis telencephali. Implications in forensic psychopathology Gheorghe S. Drăgoi1,2,*, Ileana Marinescu3 _________________________________________________________________________________________ Abstract: Topographical relief situated in the pars basalis telencephali have always been aspects of interest for anatomists and neurologists with regard to their terming and integration in neuronal systems. The main target of the present work is the macroanatonic analysis of the location and relations of reference anatomic markers of topographical relief on which connections of anatomical and functional areas are based. The study was carried out on human biological samples. Twenty samples of lesion- free encephala were taken from 8 men (aged between 36 and 65), 7 women (aged between 41 and 69) and 5 new-born infants (2 males and 3 females). Based on reference anatomic markers, identification of anatomic and functional area borderlines was done in the subcalloso – olfactory space. According to morphological variability the markers were grouped in 3 classes: a) commissural interconnective markers; b) non-commissural interconnective markers; c) markers modelled by the branches of the anterior cerebral artery. Based on our research and further corroboration with the progress made in the research of topographical relief as well as of the experimental results on septal nuclei, we offer an attempt at clarifying of the semantic content of taxonomy of terms as well as their implication in forensic phychopathology. Key Words: septum verum, subcallosal area, paraterminal gyrus, paraolfactory area, paraolfactory gyri. INTRODUCTION [7]; Cruveilhier (1836)[8]; Bergman (1831)[9]; Meynert (1867)[10]; Broca (1879)[11]; și Zuckerkandl (1887) Topographical relief variation in the pars basalis [12]. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

Patients with Stroke Confined to Basal Ganglia Have Diminished Response to Rehabilitation Efforts
Patients with stroke confined to basal ganglia have diminished response to rehabilitation efforts Ichiro Miyai, MD, PhD; Alan D. Blau, PhD; Michael J. Reding, MD; and Bruce T. Volpe, MD Article abstract-Prediction of the functional outcome for patients with stroke has depended on the severity of impair- ment, location of brain injury, age, and general medical condition. This study compared admission and discharge func- tional outcome (Functional Independence Measure, FIM) and deficit severity (Fugl-Meyer, F-M) scores in a retrospective study of patients with similar neurologic impairments: homonymous hemianopia, hemisensory loss, and hemiparesis. CT-verified stroke location was the independent variable: cortical (n = ll),basal ganglia and internal capsule (normal cortex and thalamus, n = 131, or combined (cortical, basal ganglia, and internal capsule, n = 22). By 3 months on average after stroke, all groups demonstrated significantly improved motor function as measured by F-M scores. Patients with cortical lesions had the least CT-imaged damage and the best outcome. Patients with combined lesions and more extensive brain injury had significantly higher FIM scores (p< 0.05) than patients with injury restricted to the basal ganglid internal capsule. Patients with basal ganglidinternal capsule injury were more likely to have hypotonia, flaccid paralysis, and persistently impaired balance and ambulation performance. While all patients had a comparable rehabilitation experience, these results suggest that patients with stroke confined to the basal ganglia and internal capsule benefited less from therapy. Isolated basal ganglia stroke may cause persistent corticothalamic-basal ganglia interactions that are dysfunctional and impede recovery. NEUROLOGY 1997;48:95-101 In several studies rehabilitative intervention has im- matter, but not the basal ganglia, corona radiata, or inter- proved the functional outcome of patients with nal capsule. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Differential Projection from the Motor and Limbic Cortical Regions to the Mediodorsal Thalamic Nucleus in the Dog
ACTA NEUROBIOL. EXP. 1989. 49: 23-37 DIFFERENTIAL PROJECTION FROM THE MOTOR AND LIMBIC CORTICAL REGIONS TO THE MEDIODORSAL THALAMIC NUCLEUS IN THE DOG Iwona STEPNIEWSKA and Anna KOSMAL Department of Neurophysiology, Nencki Institute of Experimental Biology 3 Pasteur Str., 02-093 Warsaw, Poland Key words: mediodorsal thalamic nucleus, motor and limbic cortices, horseradish peroxidase Abstract. The cortical afferents to the mediodorsal thalamic nucleus in the dog were studied by using horseradish peroxidase. Small injec- tions allowed to establish two specific projection zones connected sepa- rately with the lateral and medial segments of the nucleus. The lateral segment received the major projection from the dorsal half of the hemi- sphere. It included premotor and part of the motor cortices in the ante- rior sigmoid gyrus and precruciate areas as well as the presylvian cor- tex. The medial segment of the nucleus was innervated by the limbic areas of the ventral half of the hemisphere. These areas included the medloventrally located genual, subcallosal and plriform cortices, as well as the cortex of the ventral bank of the anterior rhinal sulcus and the caudal part of the orbital gyrus. The cortical fields situated between these two main cortical zones, both on the lateral and medial surfaces (rhinal and sylvian sulci and anterior cingular gyrus, respectively) sent projec- tions to both medial and lateral segments of the nucleus. These results indicate that in the mediodorsal thalamic nucleus may take place the integration of information from two functionally defined systems, the motor and limbic ones. INTRODUCTIOPJ The numerous studies of the cortical afferents of the mediodorsal thalamic nucleus (MD) showed, that although the prefrontal cortex (PFC) gives rise to the heaviest projection to MD, it is not the only cortical region projecting to thls nucleus. -

Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P
Cerebral Cortex, November 2015;25: 4351–4373 doi: 10.1093/cercor/bhv019 Advance Access Publication Date: 24 February 2015 Original Article ORIGINAL ARTICLE Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P. Aggleton1, Nicholas F. Wright1, Douglas L. Rosene2, and Richard C. Saunders3 1School of Psychology, Cardiff University, Cardiff, Wales CF10 3AT, UK, 2School of Medicine, Department of Anatomy and Neurobiology, Boston University, Boston MA 02118, USA, and 3Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda, MD 20892, USA Address correspondence to: John P. Aggleton, School of Psychology, Cardiff University, Park Place, Cardiff, Wales CF10 3AT, UK. Email: [email protected] Abstract The projections from the amygdala and hippocampus (including subiculum and presubiculum) to prefrontal cortex were compared using anterograde tracers injected into macaque monkeys (Macaca fascicularis, Macaca mulatta). Almost all prefrontal areas were found to receive some amygdala inputs. These connections, which predominantly arose from the intermediate and magnocellular basal nucleus, were particularly dense in parts of the medial and orbital prefrontal cortex. Contralateral inputs were not, however, observed. The hippocampal projections to prefrontal areas were far more restricted, being confined to the ipsilateral medial and orbital prefrontal cortex (within areas 11, 13, 14, 24a, 32, and 25). These hippocampal projections principally arose from the subiculum, with the fornix providing the sole route. Thus, while the lateral prefrontal cortex essentially receives only amygdala inputs, the orbital prefrontal cortex receives both amygdala and hippocampal inputs, though these typically target different areas. Only in medial prefrontal cortex do direct inputs from both structures terminate in common sites.