IONOSPHERE by Marcel H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CARI-7 Documentation: Radiation Transport in the Atmosphere
DOT/FAA/AM-21/5 Office of Aerospace Medicine Washington, DC 20591 CARI Documentation: Radiation Transport in the Atmosphere Kyle Copeland Civil Aerospace Medical Institute Federal Aviation Administration Oklahoma City, OK 73125Location/Address March 2021 Final Report NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. _________________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: (www.faa.gov/go/oamtechreports) Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-21/5 4. Title and Subtitle 5. Report Date CARI-7 DOCUMENTATION: RADIATION TRANSPORT IN March 2021 THE ATMOSPHERE 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Copeland, K. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Civil Aerospace Medical Institute FAA 11. Contract or Grant No. 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. Washington, DC 20591 14. Sponsoring Agency Code 15. Supplemental Notes 16. Abstract Primary cosmic radiation from both the Sun and interstellar space enters Earth's atmosphere in varying amounts. Outside of Earth's atmosphere, cosmic radiation is modulated by solar activity and Earth's magnetic field. Once the radiation enters Earth's atmosphere, it interacts with Earth's atmosphere in the same manner regardless of its point of origin (solar or galactic). -

Ozone Layer Depletion (PDF)
United States Air and Radiation EPA 430-F-10-027 Environmental Protection 6205J August 2010 Agency www.epa.gov/ozone/strathome.html Ozone Layer Depletion The stratospheric ozone layer forms a thin shield in the upper atmosphere, protecting life on Earth from the sun’s ultraviolet (UV) rays. It has been called the Earth’s sunscreen. In the 1980s, scientists found evidence that the ozone layer was being depleted. Depletion of the ozone layer results in increased UV radiation reaching the Earth’s surface, which in turn leads to a greater chance of overexposure to UV radiation and the related health effects of skin cancer, cataracts, and immune suppression. This fact sheet explains the importance of protecting the stratospheric ozone layer. What is Stratospheric Ozone? Ozone is a naturally-occurring gas that can be good or bad for your health and the environment depending on its location in the atmosphere. In the layer near the Earth’s surface—the troposphere— ground-level or “bad” ozone is an air pollutant that is a key ingredient of urban smog. But higher up, in the stratosphere, “good” ozone protects life on Earth by absorbing some of the sun’s UV rays. An easy way to remember this is the phrase “good up high, bad nearby.” Ozone Layer Depletion Compounds that contain chlorine and bromine molecules, such as methyl chloroform, halons, and chlorofluorocarbons (CFCs), are stable and have atmospheric lifetimes long enough to be transported by winds into Ozone “up high” in the stratosphere protects the Earth, while ozone close to the Earth’s surface is harmful. -

Using Temperature As the Basis, the Atmosphere Is Divided Into Four Layers
Using temperature as the basis, the atmosphere is divided into four layers. The temperature decrease in the troposphere, the bottom layer in which we live, is called the "environmental lapse rate." Its average value is 6.5°C per kilometer, a figure known as the "normal lapse rate." A temperature "inversion," in which temperatures increase with height, is sometimes observed in shallow layers in the troposphere. The thickness of the troposphere is generally greater in the tropics than in polar regions. Essentially all important weather phenomena occur in the troposphere. Beyond the troposphere lies the stratosphere; the boundary between the troposphere and stratosphere is known as the tropopause. In the stratosphere, the temperature at first remains constant to a height of about 20 kilometers (12 miles) before it begins a sharp increase due to the absorption of ultraviolet radiation from the Sun by ozone. The temperatures continue to increase until the stratopause is encountered at a height of about 50 kilometers (30 miles).In the mesosphere, the third layer, temperatures again decrease with height until the mesopause, some 80 kilometers (50 miles) above the surface.The fourth layer, the thermosphere, with no well-defined upper limit, consists of extremely rarefied air. Temperatures here increase with an increase in altitude.Besides layers defined by vertical variations in temperature, the atmosphere is often divided into two layers based on composition. The homosphere (zone of homogeneous composition), from Earth’s surface to an altitude of about 80 kilometers (50 miles), consists of air that is uniform in terms of the proportions of its component gases. -

Hohonu Volume 5 (PDF)
HOHONU 2007 VOLUME 5 A JOURNAL OF ACADEMIC WRITING This publication is available in alternate format upon request. TheUniversity of Hawai‘i is an Equal Opportunity Affirmative Action Institution. VOLUME 5 Hohonu 2 0 0 7 Academic Journal University of Hawai‘i at Hilo • Hawai‘i Community College Hohonu is publication funded by University of Hawai‘i at Hilo and Hawai‘i Community College student fees. All production and printing costs are administered by: University of Hawai‘i at Hilo/Hawai‘i Community College Board of Student Publications 200 W. Kawili Street Hilo, Hawai‘i 96720-4091 Phone: (808) 933-8823 Web: www.uhh.hawaii.edu/campuscenter/bosp All rights revert to the witers upon publication. All requests for reproduction and other propositions should be directed to writers. ii d d d d d d d d d d d d d d d d d d d d d d Table of Contents 1............................ A Fish in the Hand is Worth Two on the Net: Don’t Make me Think…different, by Piper Seldon 4..............................................................................................Abortion: Murder-Or Removal of Tissue?, by Dane Inouye 9...............................An Etymology of Four English Words, with Reference to both Grimm’s Law and Verner’s Law by Piper Seldon 11................................Artifacts and Native Burial Rights: Where do We Draw the Line?, by Jacqueline Van Blarcon 14..........................................................................................Ayahuasca: Earth’s Wisdom Revealed, by Jennifer Francisco 16......................................Beak of the Fish: What Cichlid Flocks Reveal About Speciation Processes, by Holly Jessop 26................................................................................. Climatic Effects of the 1815 Eruption of Tambora, by Jacob Smith 33...........................Columnar Joints: An Examination of Features, Formation and Cooling Models, by Mary Mathis 36.................... -
Apihelion Vs
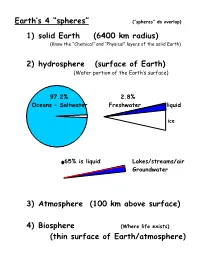
Earth’s 4 “spheres” (“spheres” do overlap) 1) solid Earth (6400 km radius) (Know the “Chemical” and “Physical” layers of the solid Earth) 2) hydrosphere (surface of Earth) (Water portion of the Earth’s surface) 97.2% 2.8% Oceans – Saltwater Freshwater liquid ice .65% is liquid Lakes/streams/air Groundwater 3) Atmosphere (100 km above surface) 4) Biosphere (Where life exists) (thin surface of Earth/atmosphere) Weather vs Climate constantly “average weather” changing 6 basic elements of weather/climate temperature of air humidity of air type & amount of cloudiness type & amount of precipitation pressure exerted by air speed & direction of wind Atmosphere Composition / Ozone Layer (pgs. 6-9) Evolution of Earth’s Atmosphere (pgs. 9-11) Exploring the Atmosphere time line for inventions/discoveries 1593 Galileo “thermometer” 1643 Torricelli barometer 1661 Boyle (P)(V)=constant 1752 Franklin kite -> lightning=electricity 1880(90) manned ballons 1900-today unmanned ballons using radiosondes = radio transmitters that send info on temperature/pressure/relative humidity today rockets & airplanes weather radar & satellites Height/Structure of Atmosphere Exosphere (above 800 km) 100 km 100 km (Ionosphere) 90 km Thermosphere 90 km 80 km 80 km 70 km 70 km 60 km Mesosphere 60 km 50 km 50 km 40 km (Ozone Layer) 40 km 30 km Stratosphere 30 km 20 km 20 km 10 km 10 km Troposphere 0 km 0 km extremely 0o hot really hot 0 100 500 1000 cold Temperature Pressure (mb) Homosphere vs Heterosphere 0-80 km above 80 km uniform distribution varies by mass of molecule N2 O He H Ionosphere located in the Thermosphere/Heterosphere N2 O ionize due to absorbing high-energy solar energy lose electrons and become +charged ions electrons are free to move Solar flares let go of lots of solar energy (charged particles) The charged particles mix with Earth’s magnetic field Charged particles are guided toward N-S magnetic poles Charged particles mix with ionosphere and cause Auroras Electromagnetic Spectrum Seasons are due to angle of sun’s rays. -

Summary of a Program Review Held at Huntsville, Alabama October 19-21, 1982
Summary of a program review held at Huntsville, Alabama October 19-21, 1982 - TECH LIBRARY KAFEI, NM lllllllsllllllRlRllffllilrml OOSSE!?b NASA Conference Publication 2259 NASA/MSFCFY-82 Atmospheric Processes Research Review Compiled by Robert E. Turner George C. Marshall Space Flight Center Marshall Space Flight Center, Alabama Summary of a program review held at Huntsville, Alabama October 19-21, 1982 National Aeronautics and Space Administration Sclontlflc and Tochnlcal InformatIon Branch 1983 ACKNOWLEDGMENTS The productive inputs and comments from the participants and attendees in the Atmospheric Processes Research Review contributed very much to the success of the review. The opportunity provided for everyone to become better acquainted with the work of other investigators and to see how the research relates to the overall objective of NASA's Atmospheric Processes Research Program was an important aspect of the review. Appreciation is expressed to all those who participated in the review. The organizers trust that participation will provide each with a better frame of reference from which to proceed with the next year's research activities. ii PREFACE Each year NASA supports research in various disciplinary program areas. The coordination and exchange of information among those sponsored by NASA to conduct research studies are important elements of each program. The Office of Space Science and Applications and the Office of Aeronautics and Space Technology, via Announcements of Opportunity (AO), Application Notices (AN),etc., invites interested investigators throughout the country to communicate their research ideas within NASA and in institutions. The proposals in the Atmospheric Processes Research area selected and assigned to the NASA Marshall Space Flight Center's (MSFC's) Atmospheric Sciences Division for technical monitorship, together with the research efforts included in the FY-82 MSFC Research and Technology Operating Plan (RTOP1 I are the source of principal focus for the NASA/MSFC FY-82 Atmospheric Processes Research Review. -

The Stratopause Evolution During Different Types of Sudden Stratospheric Warming Event
Clim Dyn DOI 10.1007/s00382-014-2292-4 The stratopause evolution during different types of sudden stratospheric warming event Etienne Vignon · Daniel M. Mitchell Received: 18 February 2014 / Accepted: 5 August 2014 © The Author(s) 2014. This article is published with open access at Springerlink.com Abstract Recent work has shown that the vertical struc- Keywords Stratopause · Sudden stratospheric warming · ture of the Arctic polar vortex during different types of MERRA data · Polar vortex · sudden stratospheric warming (SSW) events can be very Middle atmospheric circulation distinctive. Specifically, SSWs can be classified into polar vortex displacement events or polar vortex splitting events. This paper aims to study the Arctic stratosphere during 1 Introduction such events, with a focus on the stratopause using the Mod- ern Era-Restrospective analysis for Research and Applica- The stratopause is characterised by a reversal of the atmos- tions reanalysis data set. The reanalysis dataset is compared pheric lapse rate at around 50 km (~1 hPa). While strato- against two independent satellite reconstructions for valida- spheric ozone heating is responsible for the stratopause pres- tion purposes. During vortex displacement events, the strat- ence at sunlit latitudes, westward gravity wave drag (and to opause temperature and pressure exhibit a wave-1 structure a lesser extent, stationary gravity wave drag) maintains the and are in quadrature whereas during vortex splitting events stratopause in the polar night jet (Hitchman et al. 1989). they exhibit a wave-2 structure. For both types of SSW the Indeed, the westward and stationary gravity wave (GW) temperature anomalies at the stratopause are shown to be breaking induces a mesospheric meridional flow toward the generated by ageostrophic vertical motions. -

Jet Streams Anomalies As Possible Short-Term Precursors Of
Research in Geophysics 2014; volume 4:4939 Jet streams anomalies as the existence of thermal fields, con nected with big linear structures and systems of crust Correspondence: Hong-Chun Wu, Institute of possible short-term precursors faults.2 Anomalous variations of latent heat Occupational Safety and Health, Safety of earthquakes with M 6.0 flow were observed for the 2003 M 7.6 Colima Department, Shijr City, Taipei, Taiwan. > (Mexico) earthquake.3 The atmospheric Tel. +886.2.22607600227 - Fax: +886.2.26607732. E-mail: [email protected] Hong-Chun Wu,1 Ivan N. Tikhonov2 effects of ionization are observed at the alti- 1Institute of Occupational Safety and tudes of top of the atmosphere (10-12 km). Key words: earthquake, epicenter, jet stream, pre- Health, Safety Department, Shijr City, Anomalous changes in outgoing longwave cursory anomaly. radiation were recorded at the height of 300 Taipei, Taiwan; 2Institute of Marine GPa before the 26 December 2004 M 9.0 Acknowledgments: the authors would like to Geology and Geophysics, FEB RAS; w express their most profound gratitude to the Indonesia earthquake.4 These anomalous ther- Yuzhno-Sakhalinsk, Russia National Center for Environmental Prediction of mal events occurred 4-20 days prior the earth- the USA and the National Earthquake quakes.5-8 Information Center on Earthquakes of the U.S. Local anomalous changes in the ionosphere Geological Survey for data on jet streams and 9-11 earthquakes on the Internet. Abstract over epicenters can precede earthquakes. Statistical analysis showed that these changes Dedications: the authors dedicate this work to occurred about 1-5 days before seismic the memory of Miss Cai Bi-Yu, who died in Satellite data of thermal images revealed events.12 To connect the listed above abnormal Taiwan during the 1999 earthquake with magni- the existence of thermal fields, connected with phenomena to preparation of earthquakes, tude M=7.3. -

Earth's Atmospheric Layers
Earth's atmospheric layers Earth's atmospheric layers Lesson plan (Polish) Lesson plan (English) Earth's atmospheric layers Source: licencja: CC 0, [online], dostępny w internecie: www.pixabay.pl. Link to the lesson Before you start you should know what the place of the atmosphere is in relation to the lithosphere, hydrosphere, biosphere and pedosphere; that the Earth's atmosphere is the part of the Earth and moves with it. You will learn explain the term „atmosphere”; name gases that form the air and their percentage share; name permanent and variable components of atmospheric air; name the layers of the atmosphere; discuss the role of the ozone layer; characterize the effects of the ozone hole and the greenhouse effect. Nagranie dostępne na portalu epodreczniki.pl nagranie abstraktu What layers is the atmosphere built of? In the Earth's atmosphere we distinguish 5 main layers characterized by specific features and 4 intermediate layers called pauses. The boundaries between them are conventional and change depending on the geographical latitude, terrain and season of the year. The closest one to the surface of the earth is the troposphere. Its thickness ranges from 7 km (in winter) to 10 km (in summer) above the poles, and 15‐18 km above the equator. The main feature that allows determining the boundary of the troposphere is the drop in the air temperature with an increase of about 0,6°C per 100 m. In the upper layer of the troposphere, the temperature reaches -55°C (above arctic regions) to -70°C (above equatorial regions). Above this layer there is a thin tropopause with the constant temperature, and above it there is the stratosphere extending up to a height of about 50 km, in which the air temperature rises to reach 0°C. -

Ozone: Good up High, Bad Nearby
actions you can take High-Altitude “Good” Ozone Ground-Level “Bad” Ozone •Protect yourself against sunburn. When the UV Index is •Check the air quality forecast in your area. At times when the Air “high” or “very high”: Limit outdoor activities between 10 Quality Index (AQI) is forecast to be unhealthy, limit physical exertion am and 4 pm, when the sun is most intense. Twenty minutes outdoors. In many places, ozone peaks in mid-afternoon to early before going outside, liberally apply a broad-spectrum evening. Change the time of day of strenuous outdoor activity to avoid sunscreen with a Sun Protection Factor (SPF) of at least 15. these hours, or reduce the intensity of the activity. For AQI forecasts, Reapply every two hours or after swimming or sweating. For check your local media reports or visit: www.epa.gov/airnow UV Index forecasts, check local media reports or visit: www.epa.gov/sunwise/uvindex.html •Help your local electric utilities reduce ozone air pollution by conserving energy at home and the office. Consider setting your •Use approved refrigerants in air conditioning and thermostat a little higher in the summer. Participate in your local refrigeration equipment. Make sure technicians that work on utilities’ load-sharing and energy conservation programs. your car or home air conditioners or refrigerator are certified to recover the refrigerant. Repair leaky air conditioning units •Reduce air pollution from cars, trucks, gas-powered lawn and garden before refilling them. equipment, boats and other engines by keeping equipment properly tuned and maintained. During the summer, fill your gas tank during the cooler evening hours and be careful not to spill gasoline. -

Atmospheric Gases and Air Quality
12.3SECTION Atmospheric Gases and Air Quality Key Terms + Exosphere H H criteria air contaminants 500 He- He Ionosphere O Thermosphere 1 1 1 O 1NO 1OZ 1 2 1 1 Heterosphere NZ 1O 90 Photoionization N2 O2 10-5 70 Mesosphere CO 2 Pressure (mmHg) Pressure -3 of O Photodissociation Figure 12.10 Variations in 10 50 (km)Altitude 78% N2 pressure, temperature, and 21% O CO2 O2 Stratosphere 2 the components that make 1% Ar. etc. Ozone layer up Earth’s atmosphere are 30 Homosphere summarized here. 10-1 Infer How can you explain the changes in temperature Troposphere 10 of O Photodissociation H2O with altitude? 1 150 273 300 2000 major major major components components components Temperature (˚C) Figure 12.10 summarizes information about the structure and composition of Earth’s atmosphere. Much of this information is familiar to you from earlier in this unit or from your study of science or geography in earlier grades. As you know from Boyle’s law, gases are compressible. Th us, pressure in the atmosphere decreases with altitude, and this decrease is more rapid at lower altitudes than at higher altitudes. In fact, the vast majority of the mass of the atmosphere—about 99 percent—lies within 30 km of Earth’s surface. About 90 percent of the mass of the atmosphere lies within 15 km of the surface, and about 75 percent lies within 11 km. Th e atmosphere is divided into fi ve distinct regions, based on temperature changes. You may recognize the names of some or perhaps all of these regions: the troposphere, stratosphere, mesosphere, thermosphere, and exosphere. -

Cold Plasma Diagnostics in the Jovian System
341 COLD PLASMA DIAGNOSTICS IN THE JOVIAN SYSTEM: BRIEF SCIENTIFIC CASE AND INSTRUMENTATION OVERVIEW J.-E. Wahlund(1), L. G. Blomberg(2), M. Morooka(1), M. André(1), A.I. Eriksson(1), J.A. Cumnock(2,3), G.T. Marklund(2), P.-A. Lindqvist(2) (1)Swedish Institute of Space Physics, SE-751 21 Uppsala, Sweden, E-mail: [email protected], [email protected], mats.andré@irfu.se, [email protected] (2)Alfvén Laboratory, Royal Institute of Technology, SE-100 44 Stockholm, Sweden, E-mail: [email protected], [email protected], [email protected], per- [email protected] (3)also at Center for Space Sciences, University of Texas at Dallas, U.S.A. ABSTRACT times for their atmospheres are of the order of a few The Jovian magnetosphere equatorial region is filled days at most. These atmospheres can therefore rightly with cold dense plasma that in a broad sense co-rotate with its magnetic field. The volcanic moon Io, which be termed exospheres, and their corresponding ionized expels sodium, sulphur and oxygen containing species, parts can be termed exo-ionospheres. dominates as a source for this cold plasma. The three Observations indicate that the exospheres of the three icy Galilean moons (Callisto, Ganymede, and Europa) icy Galilean moons (Europa, Ganymede and Callisto) also contribute with water group and oxygen ions. are oxygen rich [1, 2]. Several authors have also All the Galilean moons have thin atmospheres with modelled these exospheres [3, 4, 5], and the oxygen was residence times of a few days at most.