Atmospheric Gases and Air Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Temperature As the Basis, the Atmosphere Is Divided Into Four Layers
Using temperature as the basis, the atmosphere is divided into four layers. The temperature decrease in the troposphere, the bottom layer in which we live, is called the "environmental lapse rate." Its average value is 6.5°C per kilometer, a figure known as the "normal lapse rate." A temperature "inversion," in which temperatures increase with height, is sometimes observed in shallow layers in the troposphere. The thickness of the troposphere is generally greater in the tropics than in polar regions. Essentially all important weather phenomena occur in the troposphere. Beyond the troposphere lies the stratosphere; the boundary between the troposphere and stratosphere is known as the tropopause. In the stratosphere, the temperature at first remains constant to a height of about 20 kilometers (12 miles) before it begins a sharp increase due to the absorption of ultraviolet radiation from the Sun by ozone. The temperatures continue to increase until the stratopause is encountered at a height of about 50 kilometers (30 miles).In the mesosphere, the third layer, temperatures again decrease with height until the mesopause, some 80 kilometers (50 miles) above the surface.The fourth layer, the thermosphere, with no well-defined upper limit, consists of extremely rarefied air. Temperatures here increase with an increase in altitude.Besides layers defined by vertical variations in temperature, the atmosphere is often divided into two layers based on composition. The homosphere (zone of homogeneous composition), from Earth’s surface to an altitude of about 80 kilometers (50 miles), consists of air that is uniform in terms of the proportions of its component gases. -
Apihelion Vs
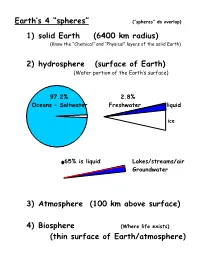
Earth’s 4 “spheres” (“spheres” do overlap) 1) solid Earth (6400 km radius) (Know the “Chemical” and “Physical” layers of the solid Earth) 2) hydrosphere (surface of Earth) (Water portion of the Earth’s surface) 97.2% 2.8% Oceans – Saltwater Freshwater liquid ice .65% is liquid Lakes/streams/air Groundwater 3) Atmosphere (100 km above surface) 4) Biosphere (Where life exists) (thin surface of Earth/atmosphere) Weather vs Climate constantly “average weather” changing 6 basic elements of weather/climate temperature of air humidity of air type & amount of cloudiness type & amount of precipitation pressure exerted by air speed & direction of wind Atmosphere Composition / Ozone Layer (pgs. 6-9) Evolution of Earth’s Atmosphere (pgs. 9-11) Exploring the Atmosphere time line for inventions/discoveries 1593 Galileo “thermometer” 1643 Torricelli barometer 1661 Boyle (P)(V)=constant 1752 Franklin kite -> lightning=electricity 1880(90) manned ballons 1900-today unmanned ballons using radiosondes = radio transmitters that send info on temperature/pressure/relative humidity today rockets & airplanes weather radar & satellites Height/Structure of Atmosphere Exosphere (above 800 km) 100 km 100 km (Ionosphere) 90 km Thermosphere 90 km 80 km 80 km 70 km 70 km 60 km Mesosphere 60 km 50 km 50 km 40 km (Ozone Layer) 40 km 30 km Stratosphere 30 km 20 km 20 km 10 km 10 km Troposphere 0 km 0 km extremely 0o hot really hot 0 100 500 1000 cold Temperature Pressure (mb) Homosphere vs Heterosphere 0-80 km above 80 km uniform distribution varies by mass of molecule N2 O He H Ionosphere located in the Thermosphere/Heterosphere N2 O ionize due to absorbing high-energy solar energy lose electrons and become +charged ions electrons are free to move Solar flares let go of lots of solar energy (charged particles) The charged particles mix with Earth’s magnetic field Charged particles are guided toward N-S magnetic poles Charged particles mix with ionosphere and cause Auroras Electromagnetic Spectrum Seasons are due to angle of sun’s rays. -

Elemental Geosystems, 5E (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere
Elemental Geosystems, 5e (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere 1) Our planet and our lives are powered by A) energy derived from inside Earth. B) radiant energy from the Sun. C) utilities and oil companies. D) shorter wavelengths of gamma rays, X-rays, and ultraviolet. Answer: B 2) Which of the following is true? A) The Sun is the largest star in the Milky Way Galaxy. B) The Milky Way is part of our Solar System. C) The Sun produces energy through fusion processes. D) The Sun is also a planet. Answer: C 3) Which of the following is true about the Milky Way galaxy in which we live? A) It is a spiral-shaped galaxy. B) It is one of millions of galaxies in the universe. C) It contains approximately 400 billion stars. D) All of the above are true. E) Only A and B are true. Answer: D 4) The planetesimal hypothesis pertains to the formation of the A) universe. B) galaxy. C) planets. D) ocean basins. Answer: C 5) The flattened structure of the Milky Way is revealed by A) the constellations of the Zodiac. B) a narrow band of hazy light that stretches across the night sky. C) the alignment of the planets in the solar system. D) the plane of the ecliptic. Answer: B 6) Earth and the Sun formed specifically from A) the galaxy. B) unknown origins. C) a nebula of dust and gases. D) other planets. Answer: C 7) Which of the following is not true of stars? A) They form in great clouds of gas and dust known as nebula. -

Lecture.1.Introduction.Pdf
Lecture 1: Introduction to the Climate System Earth’s Climate System Solar forcing T mass (& radiation) The ultimate driving T & mass relation in vertical mass (& energy, weather..) Atmosphere force to Earth’s climate system is the heating from Energy T vertical stability vertical motion thunderstorm the Sun. Ocean Land The solar energy drives What are included in Earth’s climate system? Solid Earth three major cycles (energy, water, and biogeochemisty) What are the general properties of the Atmosphere? Energy, Water, and in the climate system. How about the ocean, cryosphere, and land surface? Biogeochemistry Cycles ESS200 ESS200 Prof. Jin-Yi Yu Prof. Jin-Yi Yu Thickness of the Atmosphere (from Meteorology Today) The thickness of the atmosphere is only about 2% 90% of Earth’s thickness (Earth’s 70% radius = ~6400km). Most of the atmospheric mass is confined in the lowest 100 km above the sea level. tmosphere Because of the shallowness of the atmosphere, its motions over large A areas are primarily horizontal. Typically, horizontal wind speeds are a thousands time greater than vertical wind speeds. (But the small vertical displacements of air have an important impact on ESS200 the state of the atmosphere.) ESS200 Prof. Jin-Yi Yu Prof. Jin-Yi Yu 1 Vertical Structure of the Atmosphere Composition of the Atmosphere (inside the DRY homosphere) composition temperature electricity Water vapor (0-0.25%) 80km (from Meteorology Today) ESS200 (from The Blue Planet) ESS200 Prof. Jin-Yi Yu Prof. Jin-Yi Yu Origins of the Atmosphere What Happened to H2O? When the Earth was formed 4.6 billion years ago, Earth’s atmosphere was probably mostly hydrogen (H) and helium (He) plus hydrogen The atmosphere can only hold small fraction of the mass of compounds, such as methane (CH4) and ammonia (NH3). -

Evidence of the Complexity of Aerosol Transport in the Lower Troposphere on the Namibian Coast During AEROCLO-Sa
Atmos. Chem. Phys., 19, 14979–15005, 2019 https://doi.org/10.5194/acp-19-14979-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Evidence of the complexity of aerosol transport in the lower troposphere on the Namibian coast during AEROCLO-sA Patrick Chazette1, Cyrille Flamant2, Julien Totems1, Marco Gaetani2,3, Gwendoline Smith1,3, Alexandre Baron1, Xavier Landsheere3, Karine Desboeufs3, Jean-François Doussin3, and Paola Formenti3 1Laboratoire des Sciences du Climat et de l’Environnement (LSCE), Laboratoire mixte CEA-CNRS-UVSQ, UMR CNRS 1572, CEA Saclay, 91191 Gif-sur-Yvette, France 2LATMOS/IPSL, Sorbonne Université, CNRS, UVSQ, Paris, France 3Laboratoire Interuniversitaire des Systèmes Atmosphériques (LISA) UMR CNRS 7583, Université Paris-Est-Créteil, Université de Paris, Institut Pierre Simon Laplace, Créteil, France Correspondence: Patrick Chazette ([email protected]) Received: 29 May 2019 – Discussion started: 3 June 2019 Revised: 27 October 2019 – Accepted: 28 October 2019 – Published: 11 December 2019 Abstract. The evolution of the vertical distribution and opti- ern Africa by the equatorward moving cut-off low originat- cal properties of aerosols in the free troposphere, above stra- ing from within the westerlies. All the observations show a tocumulus, is characterized for the first time over the Namib- very complex mixture of aerosols over the coastal regions of ian coast, a region where uncertainties on aerosol–cloud cou- Namibia that must be taken into account when investigating pling in climate simulations are significant. We show the high aerosol radiative effects above stratocumulus clouds in the variability of atmospheric aerosol composition in the lower southeast Atlantic Ocean. -

Lecture 1 Lecture 1 Outline of Today's Lecture Science Scientific Method
Lecture 1 Lecture 1 Outline of Today’s Lecture • What is it that defines science? • What is meteorology? • A brief history of meteorology. • What does the atmosphere do for us? • What is the atmosphere composed of? • Intro to atmospheric structure 1 2 Science Scientific Method • What is it that defines science? 1. Start with an educated guess or “hypothesis” 2. The guess should leads to a prediction that can • How is science distinguished from be tested. other academic endeavors (e.g, history 3. Design an experiment to test the hypothesis and or comparative religion)? prediction. 4. Evaluate the results of the experiment to verify or • Why is creationism not considered refute hypothesis science by scientists? 5. Construct a theory (or model) based on test results. 6. Repeat cycle (1-5) to refine/modify the theory. 3 4 Scientific Method What is Meteorology? • Our understanding of the world grows as our theories become more complete and precise. • The term meteorology comes from the Greek • A key to the Scientific Method is that the results of a word meteoros, meaning, “high in the air.” good experiment are reproducible. The same • Rain and snow are hydrometeors. experiment using the same hardware will produce the same results time after time. • Meteorology is the study of the atmosphere • If a hypothesis can not be tested then it falls outside the and the processes that produce weather. current realm of scientific understanding or knowledge, • Meteorology is also called atmospheric and is considered “speculation.” science. 5 6 A Brief History of Meteorology A Brief History of Meteorology 340 BC In a book he called Meteorologica, the Greek Answer: Lack of instruments to make observations. -

Environmental Structure and Function: Earth System - Nikita Glazovsky
EARTH SYSTEM: HISTORY AND NATURAL VARIABILITY - Vol. IV - Environmental Structure and Function: Earth System - Nikita Glazovsky ENVIRONMENTAL STRUCTURE AND FUNCTION: EARTH SYSTEM Nikita Glazovsky Institute of Geography, Russian Academy of Sciences (RAS), Moscow, Russia Keywords: astenosphere, atmosphere, circulation, Coriolis effect, environment, heterosphere, air (atmospheric) pressure, weather, troposphere, stratosphere, mesosphere, cyclone, anticyclone, cloudiness, radiation, geosphere, geostrophic wind, gradient wind, greenhouse effect, hydrosphere, homosphere, solar radiation, heat radiation, hydrosphere, freshwater, sea level, groundwater, sea water, snow cover, permafrost, river, lake, glacier, glaciation, ice sheet, lithosphere, geological process, mineral resources, land forms, pedosphere, soil genesis, geography, landscape, variability, water cycle Contents 1. Introduction 2. Atmosphere 2.1. Composition and Structure of the Atmosphere 2.2. Physics of the Atmosphere 2.3. Chemistry of the Atmosphere 2.4. The Atmosphere as a Colloidal Medium 2.5. Nature of Atmospheric Circulation 2.6. Modeling of Atmospheric Circulation 3. Hydrosphere 3.1. Atmospheric Waters 3.2. Hydrological Cycle on the Earth 3.3. Surface Water 3.4. Ice in the Hydrosphere 3.5. Underground Water 3.6. Oceans 3.7. Water of Living Organisms 4. Cryosphere 4.1. Ice in theUNESCO Atmosphere – EOLSS 4.2. Glaciosphere 4.3. Glaciers 4.4. Sea ice 4.5. Snow CoverSAMPLE CHAPTERS 4.6. Cryolithozone (Cryolithic Zone) 5. Lithosphere 6. Pedosphere 6.1. Soil Components 6.2. Vertical Structure of Soils 6.3. Soil-Forming Factors 6.4. Age of Soils 6.5. Types of Soils 6.6. Importance of Soils ©Encyclopedia of Life Support Systems (EOLSS) EARTH SYSTEM: HISTORY AND NATURAL VARIABILITY - Vol. IV - Environmental Structure and Function: Earth System - Nikita Glazovsky 7. -

Earth System Science 5 : Homework #1 (Due 4/10/2008)
Earth System Science 5 : Homework #1 (Due 4/10/2008) Name___________________________________ Student ID: ______________________________ 1) The temperature is lowest here: 6) In this atmospheric layer, the temperature is A) mesopause. B) stratosphere. relatively constant for the first 10 kilometers, then it increases: C) tropopause. D) stratopause. A) troposphere. B) mesosphere. Answer: A C) stratosphere. D) thermosphere. 2) The atmosphere is a mixture of: Answer: C A) precipitation and air. 7) According to Wien's law: B) gas molecules, small particulates, and moisture. A) the wavelength of peak radiation is proportional to temperature. C) moisture and cas molecules only. B) the Sun's energy intensity peaks in the D) particulate matter and water. visible portion of the electromagnetic Answer: B spectrum. C) wavelength is proportional to the fourth 3) A "greenhouse" works because: power of the intensity of radiation. A) all greenhouses face south and into the D) the radiation emitted from Earth must be maximum angle of solar energy. 4 micrometers or longer. B) of the difference in the solar constant. Answer: B C) the windows of the greenhouse only allow green light wavelengths to pass 8) The largest energy transfer in the solar through. spectrum occurs in the: D) short wave lengths of energy pass A) radio wave part of the spectrum. through the glass but longer ones can't. B) Infrared part of the spectrum. Answer: D C) visible part of the spectrum. D) ultraviolet part of the spectrum. 4) Albedo: E) x-ray part of the spectrum. A) is high for sand and dirt. B) is high for ice, snow and thick clouds. -

Troposphere on the Namibian Coast During AEROCLO-Sa
https://doi.org/10.5194/acp-2019-507 Preprint. Discussion started: 3 June 2019 c Author(s) 2019. CC BY 4.0 License. 1 Evidence of the complexity of aerosol transport in the lower 2 troposphere on the Namibian coast during AEROCLO-sA 3 Patrick Chazette1, Cyrille Flamant2, Julien Totems1, Marco Gaetani2,3, Gwendoline Smith1,3, 4 Alexandre Baron1, Xavier Landsheere3, Karine Desboeufs3, Jean-François Doussin3, and Paola 5 Formenti3 6 1Laboratoire des Sciences du Climat et de l’Environnement (LSCE), Laboratoire mixte CEA-CNRS-UVSQ, UMR 7 CNRS 1572, CEA Saclay, 91191 Gif-sur-Yvette, France 8 2LATMOS/IPSL, Sorbonne Université, CNRS, UVSQ, Paris, France 9 3 Laboratoire Interuniversitaire des Systèmes Atmosphériques (LISA) UMR CNRS 7583, Université Paris-Est- 10 Créteil, Université de Paris, Institut Pierre Simon Laplace, Créteil, France. 11 Correspondence to: Patrick Chazette ([email protected]) 12 Abstract. The evolution of the vertical distribution and optical properties of aerosols in the free troposphere, above 13 stratocumulus, is analysed for the first time over the Namibian coast, a region where uncertainties on aerosol-cloud 14 coupling in climate simulations are significant. We show the high variability of atmospheric aerosol composition 15 in the lower and middle troposphere during the AEROCLO-sA field campaign (22 August - 12 September 2017) 16 around the Henties Bay supersite, using a combination of ground-based, airborne and space-borne lidar 17 measurements. Three distinct periods of 4 to 7 days are observed, associated with increasing aerosol loads (aerosol 18 optical thickness at 550 nm ranging from ~ 0.2 to ~0.7), as well as increasing aerosol layer depth and top altitude. -

Evidence of the Complexity of Aerosol Transport in the Lower Troposphere
1 Evidence of the complexity of aerosol transport in the lower 2 troposphere on the Namibian coast during AEROCLO-sA 3 Patrick Chazette1, Cyrille Flamant2, Julien Totems1, Marco Gaetani2,3, Gwendoline Smith1,3, 4 Alexandre Baron1, Xavier Landsheere3, Karine Desboeufs3, Jean-François Doussin3, and Paola 5 Formenti3 6 1Laboratoire des Sciences du Climat et de l’Environnement (LSCE), Laboratoire mixte CEA-CNRS-UVSQ, UMR 7 CNRS 1572, CEA Saclay, 91191 Gif-sur-Yvette, France 8 2LATMOS/IPSL, Sorbonne Université, CNRS, UVSQ, Paris, France 9 3 Laboratoire Interuniversitaire des Systèmes Atmosphériques (LISA) UMR CNRS 7583, Université Paris-Est- 10 Créteil, Université de Paris, Institut Pierre Simon Laplace, Créteil, France. 11 Correspondence to: Patrick Chazette ([email protected]) 12 Abstract. The evolution of the vertical distribution and optical properties of aerosols in the free troposphere, above 13 stratocumulus, is characterized for the first time over the Namibian coast, a region where uncertainties on aerosol- 14 cloud coupling in climate simulations are significant. We show the high variability of atmospheric aerosol 15 composition in the lower and middle troposphere during the AEROCLO-sA field campaign (22 August - 12 16 September 2017) around the Henties Bay supersite, using a combination of ground-based, airborne and space- 17 borne lidar measurements. Three distinct periods of 4 to 7 days are observed, associated with increasing aerosol 18 loads (aerosol optical thickness at 550 nm ranging from ~ 0.2 to ~0.7), as well as increasing lofted aerosol layer 19 depth and top altitude. Aerosols are observed up to 6 km above mean sea level during the later period. -

Elemental Geosystems, 5E (Christopherson) Chapter 1 Foundations of Geography
Elemental Geosystems, 5e (Christopherson) Chapter 1 Foundations of Geography 1) Geography is described as A) an Earth science. B) a human science. C) a physical science. D) a spatial science. Answer: D 2) The word spatial refers to A) the nature and character of physical space. B) items that relate specifically to society. C) things that are unique and special. D) eras of time. Answer: A 3) A principal methodology governing geographic inquiry A) is behavioral analysis. B) involves spatial analysis. C) uses chronological organization. D) is field work. Answer: B 4) Which of the following best describes the current emphasis in the field of physical geography? A) understanding soil development B) modeling economic interrelationships among countries C) studying weather D) understanding how Earth's systems interact to produce natural phenomena Answer: D 5) Which of the following most accurately characterizes the goal of geography? A) the production of maps B) memorization of the names of places on world and regional maps C) memorization of the imports and exports of a country D) understanding why a place has the characteristics that it does Answer: D 6) Which of the following terms characterizes the discipline of geography? A) eclectic B) holistic C) unscientific D) A, B, and C E) A and B only Answer: E 7) Which of the following compose the fundamental duality in the field of geography? A) physical versus human/cultural B) physical versus economic C) economic versus political D) political versus environmental 1 Answer: A 8) Relative to the fundamental themes of geography proposed by the Association of American Geographers, communication and diffusion refer to A) location. -

Lecture 1 Atmosphere, Ocean, and Land Surface
Lecture 1 Atmosphere, Ocean, and Land Surface Nomenclature: The west-to-east direction is referred to as the zonal direction and the south-to-north direction is referred to as the meridional direction. Earth’s atmosphere NASA GSFC Graphic The vertical temperature profile - Standard Atmosphere The average surface temperature of Earth is ~ 288K. THERMOSPHERE 90 mesopause In the stratosphere air warms 80 up as ozone (O3) absorbs MESOSPHERE 70 (.1%) ultra-violet (UV) radiation. 60 homosphere 50 stratopause 40 STRATOSPHERE In the troposphere the altitude (km) ozone (9%) 30 layer temperature decreases about 20 tropopause 7K per kilometer. 10 TROPOSPHERE (90%) 0 150 200 250 300 temperature (K) Fig. 1.8 Vertical distributions of air pressure and partial pressure of water vapor as functions of altitude for globally and annually averaged conditions. Values have been normalized by dividing by the surface values of 1013.25 and 17.5 mb (millibars), respectively. (Hartmann’s) Atmospheric Temperature Fig. 1.3 Annual-mean temperature profiles for the lowest 20 km of the atmosphere in three latitude bands. (Hartmann’s) Atmospheric Temperature Fig. 1.4 Seasonal variation of temperature profiles at 75 degrees North (Hartmann’s) The vertical temperature profile in the upper ocean The surface layer of TEMPERATURE PROFILE IN THE OCEAN (!45°N) the ocean where 4°C 10°C 16°C temperatures are about constant in the Mixed layer vertical is known as 20 m May August the oceanic mixed 40 m layer. 60 m Further down, the temperature 80 m decreases with 100 m depth in the layer March known as the thermocline.