Lecture.1.Introduction.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Temperature As the Basis, the Atmosphere Is Divided Into Four Layers
Using temperature as the basis, the atmosphere is divided into four layers. The temperature decrease in the troposphere, the bottom layer in which we live, is called the "environmental lapse rate." Its average value is 6.5°C per kilometer, a figure known as the "normal lapse rate." A temperature "inversion," in which temperatures increase with height, is sometimes observed in shallow layers in the troposphere. The thickness of the troposphere is generally greater in the tropics than in polar regions. Essentially all important weather phenomena occur in the troposphere. Beyond the troposphere lies the stratosphere; the boundary between the troposphere and stratosphere is known as the tropopause. In the stratosphere, the temperature at first remains constant to a height of about 20 kilometers (12 miles) before it begins a sharp increase due to the absorption of ultraviolet radiation from the Sun by ozone. The temperatures continue to increase until the stratopause is encountered at a height of about 50 kilometers (30 miles).In the mesosphere, the third layer, temperatures again decrease with height until the mesopause, some 80 kilometers (50 miles) above the surface.The fourth layer, the thermosphere, with no well-defined upper limit, consists of extremely rarefied air. Temperatures here increase with an increase in altitude.Besides layers defined by vertical variations in temperature, the atmosphere is often divided into two layers based on composition. The homosphere (zone of homogeneous composition), from Earth’s surface to an altitude of about 80 kilometers (50 miles), consists of air that is uniform in terms of the proportions of its component gases. -
Apihelion Vs
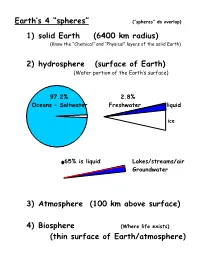
Earth’s 4 “spheres” (“spheres” do overlap) 1) solid Earth (6400 km radius) (Know the “Chemical” and “Physical” layers of the solid Earth) 2) hydrosphere (surface of Earth) (Water portion of the Earth’s surface) 97.2% 2.8% Oceans – Saltwater Freshwater liquid ice .65% is liquid Lakes/streams/air Groundwater 3) Atmosphere (100 km above surface) 4) Biosphere (Where life exists) (thin surface of Earth/atmosphere) Weather vs Climate constantly “average weather” changing 6 basic elements of weather/climate temperature of air humidity of air type & amount of cloudiness type & amount of precipitation pressure exerted by air speed & direction of wind Atmosphere Composition / Ozone Layer (pgs. 6-9) Evolution of Earth’s Atmosphere (pgs. 9-11) Exploring the Atmosphere time line for inventions/discoveries 1593 Galileo “thermometer” 1643 Torricelli barometer 1661 Boyle (P)(V)=constant 1752 Franklin kite -> lightning=electricity 1880(90) manned ballons 1900-today unmanned ballons using radiosondes = radio transmitters that send info on temperature/pressure/relative humidity today rockets & airplanes weather radar & satellites Height/Structure of Atmosphere Exosphere (above 800 km) 100 km 100 km (Ionosphere) 90 km Thermosphere 90 km 80 km 80 km 70 km 70 km 60 km Mesosphere 60 km 50 km 50 km 40 km (Ozone Layer) 40 km 30 km Stratosphere 30 km 20 km 20 km 10 km 10 km Troposphere 0 km 0 km extremely 0o hot really hot 0 100 500 1000 cold Temperature Pressure (mb) Homosphere vs Heterosphere 0-80 km above 80 km uniform distribution varies by mass of molecule N2 O He H Ionosphere located in the Thermosphere/Heterosphere N2 O ionize due to absorbing high-energy solar energy lose electrons and become +charged ions electrons are free to move Solar flares let go of lots of solar energy (charged particles) The charged particles mix with Earth’s magnetic field Charged particles are guided toward N-S magnetic poles Charged particles mix with ionosphere and cause Auroras Electromagnetic Spectrum Seasons are due to angle of sun’s rays. -

Exoplanet Atmospheres and Interiors Types of Planets
Exoplanet Atmospheres and Interiors Types of Planets • Hot Jupiters – Most common, because of observaonal biases – Temperatures ~ 1000K • Neptunes – May resemble the gas giants in our solar system • Super-earths – Rocky? – Water planets? • Terrestrial Atmosphere Basics • Ρ(h) = P(0) e-h/h0 • h0=kT/mg – k = Boltzmann constant – T=temperature – m=mass of par?cle – g=gravitaonal acceleraon • Density falls off exponen?ally with height Atmospheric Complicaons Stars have relavely simple atmospheres • A few diatomic molecules Brown Dwarfs and Planets are more complex • Temperature Inversions (external heang) • Clouds – Probably exist in T dwarfs – precipitaon • Molecules • Chemistry Generalized Thermal Atmosphere • Blue regions are convecve = lapse rate Marley, ARAA, fig 1 Atmospheric Chemistry • Ionizaon equilibrium • Chemical equilibrium High T Low T Low P High P Marley, ARAA, Fig 4 Marley, ARAA, Fig 3 • 2 MJ planet, T=600K, g=10 Marley, ARAA, Fig 5 Condensaon Sequence Transit Photometry Transit widths may reveal high al?tude haze 2 2 Excess eclipse depth δ ≃ ((Rp+ nH)/R∗) – (Rp/R∗) H = pressure scale height n = number of scale heights For typical hot Jupiters, δ ≅ 0.1% Observaons: Photometry • Precision photometry of transi?ng planet can give temperature – Comparison with Teq gives albedo (for Rp) • Inference of Na D1,D2 lines in HD 209458: – Eclipse 0.0002 deeper in lines – Charbonneau et al 2002, ApJ, 568, 377 Observaons: Photometry • Precision photometry of transi?ng planet can give temperature 2 2 – fin = (1-A) L* πRp / 4πd 2 4 – fout = 4πRp σTp – Comparison with Teq gives albedo (for Rp) For A=0: TP=T*√(R*/2d) Hot Jupiters • A is generally small: no clouds • Phase curve -> atmospheric dynamics • Flux variaons -> day to night temperature variaons • Hot Jupiters expected to have jet streams Basic Atmospheric Circulaon – no rotaon (Hadley cells) Jupiter HD 189733b • Temperature: 970-1200K • Peak 30o East • Winds up to 8700 km/h • H atm. -

Atmospheric Gases and Air Quality
12.3SECTION Atmospheric Gases and Air Quality Key Terms + Exosphere H H criteria air contaminants 500 He- He Ionosphere O Thermosphere 1 1 1 O 1NO 1OZ 1 2 1 1 Heterosphere NZ 1O 90 Photoionization N2 O2 10-5 70 Mesosphere CO 2 Pressure (mmHg) Pressure -3 of O Photodissociation Figure 12.10 Variations in 10 50 (km)Altitude 78% N2 pressure, temperature, and 21% O CO2 O2 Stratosphere 2 the components that make 1% Ar. etc. Ozone layer up Earth’s atmosphere are 30 Homosphere summarized here. 10-1 Infer How can you explain the changes in temperature Troposphere 10 of O Photodissociation H2O with altitude? 1 150 273 300 2000 major major major components components components Temperature (˚C) Figure 12.10 summarizes information about the structure and composition of Earth’s atmosphere. Much of this information is familiar to you from earlier in this unit or from your study of science or geography in earlier grades. As you know from Boyle’s law, gases are compressible. Th us, pressure in the atmosphere decreases with altitude, and this decrease is more rapid at lower altitudes than at higher altitudes. In fact, the vast majority of the mass of the atmosphere—about 99 percent—lies within 30 km of Earth’s surface. About 90 percent of the mass of the atmosphere lies within 15 km of the surface, and about 75 percent lies within 11 km. Th e atmosphere is divided into fi ve distinct regions, based on temperature changes. You may recognize the names of some or perhaps all of these regions: the troposphere, stratosphere, mesosphere, thermosphere, and exosphere. -

A Featureless Transmission Spectrum for the Neptune-Mass Exoplanet GJ 436B
A featureless transmission spectrum for the Neptune-mass exoplanet GJ 436b Heather A. Knutson1, Björn Benneke1,2, Drake Deming3, & Derek Homeier4 1Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125, USA. 2Department of Earth, Atmospheric, and Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. 3Department of Astronomy, University of Maryland, College Park, MD 20742, USA. 4Centre de Recherche Astrophysique de Lyon, 69364 Lyon, France. GJ 436b is a warm (approximately 800 K) extrasolar planet that periodically eclipses its low-mass (0.5 MSun) host star, and is one of the few Neptune-mass planets that is amenable to detailed characterization. Previous observations1,2,3 have indicated that its atmosphere has a methane-to-CO ratio that is 105 times smaller than predicted by models for hydrogen-dominated atmospheres at these temperatures4,5. A recent study proposed that this unusual chemistry could be explained if the planet’s atmosphere is significantly enhanced in elements heavier than H and He6. In this study we present complementary observations of GJ 436b’s atmosphere obtained during transit. Our observations indicate that the planet’s transmission spectrum is effectively featureless, ruling out cloud-free, hydrogen-dominated atmosphere models with a significance of 48σ. The measured spectrum is consistent with either a high cloud or haze layer located at a pressure of approximately 1 mbar or with a relatively hydrogen-poor (3% H/He mass fraction) atmospheric composition7,8,9. We observed four transits of the Neptune-mass planet GJ 436b on UT Oct 26, Nov 29, and Dec 10 2012, and Jan 2 2013 using the red grism (1.2-1.6 µm) on the Hubble Space Telescope (HST) Wide Field Camera 3 instrument. -

REVIEW Doi:10.1038/Nature13782
REVIEW doi:10.1038/nature13782 Highlights in the study of exoplanet atmospheres Adam S. Burrows1 Exoplanets are now being discovered in profusion. To understand their character, however, we require spectral models and data. These elements of remote sensing can yield temperatures, compositions and even weather patterns, but only if significant improvements in both the parameter retrieval process and measurements are made. Despite heroic efforts to garner constraining data on exoplanet atmospheres and dynamics, reliable interpretation has frequently lagged behind ambition. I summarize the most productive, and at times novel, methods used to probe exoplanet atmospheres; highlight some of the most interesting results obtained; and suggest various broad theoretical topics in which further work could pay significant dividends. he modern era of exoplanet research started in 1995 with the Earth-like planet requires the ability to measure transit depths 100 times discovery of the planet 51 Pegasi b1, when astronomers detected more precisely. It was not long before many hundreds of gas giants were the periodic radial-velocity Doppler wobble in its star, 51 Peg, detected both in transit and by the radial-velocity method, the former Tinduced by the planet’s nearly circular orbit. With these data, and requiring modest equipment and the latter requiring larger telescopes knowledge of the star, the orbital period (P) and semi-major axis (a) with state-of-the-art spectrometers with which to measure the small could be derived, and the planet’s mass constrained. However, the incli- stellar wobbles. Both techniques favour close-in giants, so for many nation of the planet’s orbit was unknown and, therefore, only a lower years these objects dominated the bestiary of known exoplanets. -

Cold Plasma Diagnostics in the Jovian System
341 COLD PLASMA DIAGNOSTICS IN THE JOVIAN SYSTEM: BRIEF SCIENTIFIC CASE AND INSTRUMENTATION OVERVIEW J.-E. Wahlund(1), L. G. Blomberg(2), M. Morooka(1), M. André(1), A.I. Eriksson(1), J.A. Cumnock(2,3), G.T. Marklund(2), P.-A. Lindqvist(2) (1)Swedish Institute of Space Physics, SE-751 21 Uppsala, Sweden, E-mail: [email protected], [email protected], mats.andré@irfu.se, [email protected] (2)Alfvén Laboratory, Royal Institute of Technology, SE-100 44 Stockholm, Sweden, E-mail: [email protected], [email protected], [email protected], per- [email protected] (3)also at Center for Space Sciences, University of Texas at Dallas, U.S.A. ABSTRACT times for their atmospheres are of the order of a few The Jovian magnetosphere equatorial region is filled days at most. These atmospheres can therefore rightly with cold dense plasma that in a broad sense co-rotate with its magnetic field. The volcanic moon Io, which be termed exospheres, and their corresponding ionized expels sodium, sulphur and oxygen containing species, parts can be termed exo-ionospheres. dominates as a source for this cold plasma. The three Observations indicate that the exospheres of the three icy Galilean moons (Callisto, Ganymede, and Europa) icy Galilean moons (Europa, Ganymede and Callisto) also contribute with water group and oxygen ions. are oxygen rich [1, 2]. Several authors have also All the Galilean moons have thin atmospheres with modelled these exospheres [3, 4, 5], and the oxygen was residence times of a few days at most. -

Elemental Geosystems, 5E (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere
Elemental Geosystems, 5e (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere 1) Our planet and our lives are powered by A) energy derived from inside Earth. B) radiant energy from the Sun. C) utilities and oil companies. D) shorter wavelengths of gamma rays, X-rays, and ultraviolet. Answer: B 2) Which of the following is true? A) The Sun is the largest star in the Milky Way Galaxy. B) The Milky Way is part of our Solar System. C) The Sun produces energy through fusion processes. D) The Sun is also a planet. Answer: C 3) Which of the following is true about the Milky Way galaxy in which we live? A) It is a spiral-shaped galaxy. B) It is one of millions of galaxies in the universe. C) It contains approximately 400 billion stars. D) All of the above are true. E) Only A and B are true. Answer: D 4) The planetesimal hypothesis pertains to the formation of the A) universe. B) galaxy. C) planets. D) ocean basins. Answer: C 5) The flattened structure of the Milky Way is revealed by A) the constellations of the Zodiac. B) a narrow band of hazy light that stretches across the night sky. C) the alignment of the planets in the solar system. D) the plane of the ecliptic. Answer: B 6) Earth and the Sun formed specifically from A) the galaxy. B) unknown origins. C) a nebula of dust and gases. D) other planets. Answer: C 7) Which of the following is not true of stars? A) They form in great clouds of gas and dust known as nebula. -

4.4 Atmospheres of Solar System Planets
4.4. ATMOSPHERES OF SOLAR SYSTEM PLANETS 87 4.4 Atmospheres of solar system planets For spectroscopic studies of planets one needs to understand the net emission of the radiation from the surface or the atmosphere. Radiative transfer in planetary atmosphere is therefore a very important topic for the analysis of solar system objects but also for direct observations of extra-solar planets. In this section we discuss some basic properties of planetary atmospheres. 4.4.1 Hydrostatic structure of atmospheres The planet structure equation from Section 3.2 apply also for planetary atmospheres. One can often make the following simplifications: – the atmosphere can be calculated in a plane-parallel geometry considering only a vertical or height dependence z, – the vertical dependence of the gravitational acceleration can often be neglected for the pressure range 10 bar – 0.01 bar and one can just use g(z)=g(z = 0) = g(R)= 2 g = GMP /RP . – the equation of state can be described by the ideal gas law ⇢kT µP P (⇢)= or ⇢(P )= , µ kT where µ is the mean particle mass (in [kg] or [g]), – a mean particle mass which is constant with height µ(z)=µ can often be used in a first approximation, – a temperature which is constant with height T (z)=T can often be used as first approximation. Pressure structure. The di↵erential equation for the pressure gradient is: dP (z) µ(z)P (z) = g(z)⇢(z)= g(z) dz − − kT(z) which yields the general solution: z 1/H (z)dz kT(z) P (z)=P e− 0 P with H (z)= . -

Constraining Exoplanet Mass from Transmission Spectroscopy Arxiv
Constraining Exoplanet Mass from Transmission Spectroscopy? Julien de Wit1∗ and Sara Seager1;2 ?This is the author’s version of the work. It is posted here by permission of the AAAS for personal use, not for redistribution. The definitive version was published in Science (Vol. 342, pp. 1473, 20 December 2013), DOI: 10.1126/science.1245450. 1Department of Earth, Atmospheric and Planetary Sciences, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. 2Department of Physics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. ∗To whom correspondence should be addressed; E-mail: [email protected]. Determination of an exoplanet’s mass is a key to understanding its basic prop- erties, including its potential for supporting life. To date, mass constraints for exoplanets are predominantly based on radial velocity (RV) measurements, which are not suited for planets with low masses, large semi-major axes, or those orbiting faint or active stars. Here, we present a method to extract an exoplanet’s mass solely from its transmission spectrum. We find good agree- ment between the mass retrieved for the hot Jupiter HD 189733b from trans- arXiv:1401.6181v1 [astro-ph.EP] 23 Jan 2014 mission spectroscopy with that from RV measurements. Our method will be able to retrieve the masses of Earth-sized and super-Earth planets using data from future space telescopes that were initially designed for atmospheric char- acterization. 1 1 Introduction With over 900 confirmed exoplanets (1) and over 2300 planetary candidates known (2), research priorities are moving from planet detection to planet characterization. In this context, a planet’s mass is a fundamental parameter because it is connected to a planet’s internal and atmospheric structure and it affects basic planetary processes such as the cooling of a planet, its plate tec- tonics (3), magnetic field generation, outgassing, and atmospheric escape. -

Evidence of the Complexity of Aerosol Transport in the Lower Troposphere on the Namibian Coast During AEROCLO-Sa
Atmos. Chem. Phys., 19, 14979–15005, 2019 https://doi.org/10.5194/acp-19-14979-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Evidence of the complexity of aerosol transport in the lower troposphere on the Namibian coast during AEROCLO-sA Patrick Chazette1, Cyrille Flamant2, Julien Totems1, Marco Gaetani2,3, Gwendoline Smith1,3, Alexandre Baron1, Xavier Landsheere3, Karine Desboeufs3, Jean-François Doussin3, and Paola Formenti3 1Laboratoire des Sciences du Climat et de l’Environnement (LSCE), Laboratoire mixte CEA-CNRS-UVSQ, UMR CNRS 1572, CEA Saclay, 91191 Gif-sur-Yvette, France 2LATMOS/IPSL, Sorbonne Université, CNRS, UVSQ, Paris, France 3Laboratoire Interuniversitaire des Systèmes Atmosphériques (LISA) UMR CNRS 7583, Université Paris-Est-Créteil, Université de Paris, Institut Pierre Simon Laplace, Créteil, France Correspondence: Patrick Chazette ([email protected]) Received: 29 May 2019 – Discussion started: 3 June 2019 Revised: 27 October 2019 – Accepted: 28 October 2019 – Published: 11 December 2019 Abstract. The evolution of the vertical distribution and opti- ern Africa by the equatorward moving cut-off low originat- cal properties of aerosols in the free troposphere, above stra- ing from within the westerlies. All the observations show a tocumulus, is characterized for the first time over the Namib- very complex mixture of aerosols over the coastal regions of ian coast, a region where uncertainties on aerosol–cloud cou- Namibia that must be taken into account when investigating pling in climate simulations are significant. We show the high aerosol radiative effects above stratocumulus clouds in the variability of atmospheric aerosol composition in the lower southeast Atlantic Ocean. -

Lecture 1 Lecture 1 Outline of Today's Lecture Science Scientific Method
Lecture 1 Lecture 1 Outline of Today’s Lecture • What is it that defines science? • What is meteorology? • A brief history of meteorology. • What does the atmosphere do for us? • What is the atmosphere composed of? • Intro to atmospheric structure 1 2 Science Scientific Method • What is it that defines science? 1. Start with an educated guess or “hypothesis” 2. The guess should leads to a prediction that can • How is science distinguished from be tested. other academic endeavors (e.g, history 3. Design an experiment to test the hypothesis and or comparative religion)? prediction. 4. Evaluate the results of the experiment to verify or • Why is creationism not considered refute hypothesis science by scientists? 5. Construct a theory (or model) based on test results. 6. Repeat cycle (1-5) to refine/modify the theory. 3 4 Scientific Method What is Meteorology? • Our understanding of the world grows as our theories become more complete and precise. • The term meteorology comes from the Greek • A key to the Scientific Method is that the results of a word meteoros, meaning, “high in the air.” good experiment are reproducible. The same • Rain and snow are hydrometeors. experiment using the same hardware will produce the same results time after time. • Meteorology is the study of the atmosphere • If a hypothesis can not be tested then it falls outside the and the processes that produce weather. current realm of scientific understanding or knowledge, • Meteorology is also called atmospheric and is considered “speculation.” science. 5 6 A Brief History of Meteorology A Brief History of Meteorology 340 BC In a book he called Meteorologica, the Greek Answer: Lack of instruments to make observations.