Lecture Note on ATMOSPHERE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Temperature As the Basis, the Atmosphere Is Divided Into Four Layers
Using temperature as the basis, the atmosphere is divided into four layers. The temperature decrease in the troposphere, the bottom layer in which we live, is called the "environmental lapse rate." Its average value is 6.5°C per kilometer, a figure known as the "normal lapse rate." A temperature "inversion," in which temperatures increase with height, is sometimes observed in shallow layers in the troposphere. The thickness of the troposphere is generally greater in the tropics than in polar regions. Essentially all important weather phenomena occur in the troposphere. Beyond the troposphere lies the stratosphere; the boundary between the troposphere and stratosphere is known as the tropopause. In the stratosphere, the temperature at first remains constant to a height of about 20 kilometers (12 miles) before it begins a sharp increase due to the absorption of ultraviolet radiation from the Sun by ozone. The temperatures continue to increase until the stratopause is encountered at a height of about 50 kilometers (30 miles).In the mesosphere, the third layer, temperatures again decrease with height until the mesopause, some 80 kilometers (50 miles) above the surface.The fourth layer, the thermosphere, with no well-defined upper limit, consists of extremely rarefied air. Temperatures here increase with an increase in altitude.Besides layers defined by vertical variations in temperature, the atmosphere is often divided into two layers based on composition. The homosphere (zone of homogeneous composition), from Earth’s surface to an altitude of about 80 kilometers (50 miles), consists of air that is uniform in terms of the proportions of its component gases. -

Atmospheric Pressure
Atmospheric pressure We all know that the atmosphere of Earth exerts a pressure on all of us. This pressure is the result of a column of air bearing down on us. However, in the seventeenth century, many scientists and philosophers believed that the air had no weight, which we already proved to be untrue in the lab (Remembered the fun you had sucking air out of the POM bottle?). Evangelista Torricelli, a student of Galileo’s, proved that air has weight using another experiment. He took a glass tube longer than 760 mm that is closed at one end and filled it completely with mercury. When he inverted the tube into a dish of mercury, some of the mercury flows out, but a column of mercury remained inside the tube. Torricelli argued that the mercury surface in the dish experiences the force of Earth’s atmosphere due to gravity, which held up the column of mercury. The force exerted by the atmosphere, which depends on the atmospheric pressure, equals the weight of mercury column in the tube. Therefore, the height of the mercury column can be used as a measure of atmospheric pressure. Although Torricelli’s explanation met with fierce opposition, it also had supporters. Blaise Pascal, for example, had one of Torricelli’s barometers carried to the top of a mountain and compared its reading there with the reading on a duplicate barometer at the base of the mountain. As the barometer was carried up, the height of the mercury column decreased, as expected, because the amount of air pressing down on the mercury in the dish decreased as the instrument was carried higher. -
Apihelion Vs
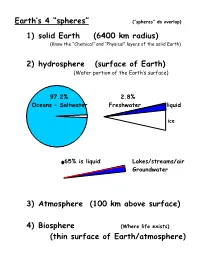
Earth’s 4 “spheres” (“spheres” do overlap) 1) solid Earth (6400 km radius) (Know the “Chemical” and “Physical” layers of the solid Earth) 2) hydrosphere (surface of Earth) (Water portion of the Earth’s surface) 97.2% 2.8% Oceans – Saltwater Freshwater liquid ice .65% is liquid Lakes/streams/air Groundwater 3) Atmosphere (100 km above surface) 4) Biosphere (Where life exists) (thin surface of Earth/atmosphere) Weather vs Climate constantly “average weather” changing 6 basic elements of weather/climate temperature of air humidity of air type & amount of cloudiness type & amount of precipitation pressure exerted by air speed & direction of wind Atmosphere Composition / Ozone Layer (pgs. 6-9) Evolution of Earth’s Atmosphere (pgs. 9-11) Exploring the Atmosphere time line for inventions/discoveries 1593 Galileo “thermometer” 1643 Torricelli barometer 1661 Boyle (P)(V)=constant 1752 Franklin kite -> lightning=electricity 1880(90) manned ballons 1900-today unmanned ballons using radiosondes = radio transmitters that send info on temperature/pressure/relative humidity today rockets & airplanes weather radar & satellites Height/Structure of Atmosphere Exosphere (above 800 km) 100 km 100 km (Ionosphere) 90 km Thermosphere 90 km 80 km 80 km 70 km 70 km 60 km Mesosphere 60 km 50 km 50 km 40 km (Ozone Layer) 40 km 30 km Stratosphere 30 km 20 km 20 km 10 km 10 km Troposphere 0 km 0 km extremely 0o hot really hot 0 100 500 1000 cold Temperature Pressure (mb) Homosphere vs Heterosphere 0-80 km above 80 km uniform distribution varies by mass of molecule N2 O He H Ionosphere located in the Thermosphere/Heterosphere N2 O ionize due to absorbing high-energy solar energy lose electrons and become +charged ions electrons are free to move Solar flares let go of lots of solar energy (charged particles) The charged particles mix with Earth’s magnetic field Charged particles are guided toward N-S magnetic poles Charged particles mix with ionosphere and cause Auroras Electromagnetic Spectrum Seasons are due to angle of sun’s rays. -

Characterising the Three-Dimensional Ozone Distribution of a Tidally Locked Earth-Like Planet
Proedrou and Hocke Earth, Planets and Space (2016) 68:96 DOI 10.1186/s40623-016-0461-x FULL PAPER Open Access Characterising the three‑dimensional ozone distribution of a tidally locked Earth‑like planet Elisavet Proedrou1,2*† and Klemens Hocke1,2,3† Abstract We simulate the 3D ozone distribution of a tidally locked Earth-like exoplanet using the high-resolution, 3D chemistry- climate model CESM1(WACCM) and study how the ozone layer of a tidally locked Earth (TLE) (�TLE = 1/365 days) differs from that of our present-day Earth (PDE) �( PDE = 1/1 day). The middle atmosphere reaches a steady state asymptotically within the first 80 days of the simulation. An upwelling, centred on the subsolar point, is present on the day side while a downwelling, centred on the antisolar point, is present on the night side. In the mesosphere, we find similar global ozone distributions for the TLE and the PDE, with decreased ozone on the day side and enhanced ozone on the night side. In the lower mesosphere, a jet stream transitions into a large-scale vortex around a low-pressure system, located at low latitudes of the TLE night side. In the middle stratosphere, the concentration of odd oxygen is approximately equal to that of the ozone [(Ox) ≈ (O3)]. At these altitudes, the lifetime of odd oxygen is ∼16 h and the transport processes significantly contribute to the global distribution of stratospheric ozone. Compared to the PDE, where the strong Coriolis force acts as a mixing barrier between low and high latitudes, the transport processes of the TLE are governed by jet streams variable in the zonal and meridional directions. -

Atmospheric Gases and Air Quality
12.3SECTION Atmospheric Gases and Air Quality Key Terms + Exosphere H H criteria air contaminants 500 He- He Ionosphere O Thermosphere 1 1 1 O 1NO 1OZ 1 2 1 1 Heterosphere NZ 1O 90 Photoionization N2 O2 10-5 70 Mesosphere CO 2 Pressure (mmHg) Pressure -3 of O Photodissociation Figure 12.10 Variations in 10 50 (km)Altitude 78% N2 pressure, temperature, and 21% O CO2 O2 Stratosphere 2 the components that make 1% Ar. etc. Ozone layer up Earth’s atmosphere are 30 Homosphere summarized here. 10-1 Infer How can you explain the changes in temperature Troposphere 10 of O Photodissociation H2O with altitude? 1 150 273 300 2000 major major major components components components Temperature (˚C) Figure 12.10 summarizes information about the structure and composition of Earth’s atmosphere. Much of this information is familiar to you from earlier in this unit or from your study of science or geography in earlier grades. As you know from Boyle’s law, gases are compressible. Th us, pressure in the atmosphere decreases with altitude, and this decrease is more rapid at lower altitudes than at higher altitudes. In fact, the vast majority of the mass of the atmosphere—about 99 percent—lies within 30 km of Earth’s surface. About 90 percent of the mass of the atmosphere lies within 15 km of the surface, and about 75 percent lies within 11 km. Th e atmosphere is divided into fi ve distinct regions, based on temperature changes. You may recognize the names of some or perhaps all of these regions: the troposphere, stratosphere, mesosphere, thermosphere, and exosphere. -

What Is Ozone Layer? a Layer in the Atmosphere of Earth That Protects Us from Harmful UV Rays from the Sun
International Day for Preservation of Ozone Layer 16 September 2020 Image Source: https://sfxstl.org/circleofcreation What is Ozone layer? A layer in the atmosphere of Earth that protects us from harmful UV rays from the Sun. It’s responsible for preserving life on the planet! What is happening? The ozone layer has begun to deplete because of harmful chemical substances and gases. This problem is not only contributing to global warming and climate change, but also allowing the dangerous radiation from the sun to affect human beings and ecosystems! Source: https://www.un.org/en/events/ozoneday/ What is causing its depletion? ● Human activities are the biggest cause of the ozone layer depletion ● When we burn coal, natural gas and other fuels for electricity, they release harmful gases such as carbon dioxide, nitrous oxide, etc that spread into the atmosphere and surround us like a blanket ● These harmful gases, called greenhouse gases, trap heat and radiation from the sun, which is causing the depletion as well as global warming. Clorofluorocarbons (CFCs), which are found in ACs and halogens, are other harmful greenhouse gases. Sources: https://e360.yale.edu/features/geoengineer-the-planet-more-scientists-now-say-it-must-be-an-option https://www.ucsusa.org/resources/ozone-hole-and-global-warming What can we do? Some things we can do to reduce our contribution to ozone layer depletion are: 1. Minimize use of cars. 2. Maintain your ACs regularly. 3. Avoid using products that are harmful to the environment and to us. 4. Buy local products which are more eco- friendly. -

A Study on the Concentration and Dispersion of Pm10 in Uthm by Using Simple Modelling and Meteorological Factors
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UTHM Institutional Repository i A STUDY ON THE CONCENTRATION AND DISPERSION OF PM10 IN UTHM BY USING SIMPLE MODELLING AND METEOROLOGICAL FACTORS MALEK FAIZAL B ABD RAHMAN A Project Report submitted in partial fulfilment of the requirements for the award of the Degree of Master of Engineering in Civil Engineering Faculty of Civil and Environmental Engineering Universiti Tun Hussein Onn Malaysia FEBRUARY, 2013 v ABSTRACT Air pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere. Air pollution can also be known as degradation of air quality resulting from unwanted chemicals or other materials occurring in the air. The simple way to know how polluted the air is to calculate the amounts of foreign and/or natural substances occurring in the atmosphere that may result in adverse effects to humans, animals, vegetation and/or materials. The objective of this study is to create a simulation of air quality dispersion in UTHM campus by using computer aided design mechanism such as software and calculating tools. Another objective is to compare the concentration obtained from the end result of calculation with past studies. The air pollutant in the scope of study is Particulate Matter (PM10). The highest reading recorded for E-Sampler was 305µg/m3. It was recorded in Structure Lab sampling point while the highest expected concentration by the Gaussian Dispersion Model was 184µg/m3 for UTHM Stadium. -

Elemental Geosystems, 5E (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere
Elemental Geosystems, 5e (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere 1) Our planet and our lives are powered by A) energy derived from inside Earth. B) radiant energy from the Sun. C) utilities and oil companies. D) shorter wavelengths of gamma rays, X-rays, and ultraviolet. Answer: B 2) Which of the following is true? A) The Sun is the largest star in the Milky Way Galaxy. B) The Milky Way is part of our Solar System. C) The Sun produces energy through fusion processes. D) The Sun is also a planet. Answer: C 3) Which of the following is true about the Milky Way galaxy in which we live? A) It is a spiral-shaped galaxy. B) It is one of millions of galaxies in the universe. C) It contains approximately 400 billion stars. D) All of the above are true. E) Only A and B are true. Answer: D 4) The planetesimal hypothesis pertains to the formation of the A) universe. B) galaxy. C) planets. D) ocean basins. Answer: C 5) The flattened structure of the Milky Way is revealed by A) the constellations of the Zodiac. B) a narrow band of hazy light that stretches across the night sky. C) the alignment of the planets in the solar system. D) the plane of the ecliptic. Answer: B 6) Earth and the Sun formed specifically from A) the galaxy. B) unknown origins. C) a nebula of dust and gases. D) other planets. Answer: C 7) Which of the following is not true of stars? A) They form in great clouds of gas and dust known as nebula. -

Lecture.1.Introduction.Pdf
Lecture 1: Introduction to the Climate System Earth’s Climate System Solar forcing T mass (& radiation) The ultimate driving T & mass relation in vertical mass (& energy, weather..) Atmosphere force to Earth’s climate system is the heating from Energy T vertical stability vertical motion thunderstorm the Sun. Ocean Land The solar energy drives What are included in Earth’s climate system? Solid Earth three major cycles (energy, water, and biogeochemisty) What are the general properties of the Atmosphere? Energy, Water, and in the climate system. How about the ocean, cryosphere, and land surface? Biogeochemistry Cycles ESS200 ESS200 Prof. Jin-Yi Yu Prof. Jin-Yi Yu Thickness of the Atmosphere (from Meteorology Today) The thickness of the atmosphere is only about 2% 90% of Earth’s thickness (Earth’s 70% radius = ~6400km). Most of the atmospheric mass is confined in the lowest 100 km above the sea level. tmosphere Because of the shallowness of the atmosphere, its motions over large A areas are primarily horizontal. Typically, horizontal wind speeds are a thousands time greater than vertical wind speeds. (But the small vertical displacements of air have an important impact on ESS200 the state of the atmosphere.) ESS200 Prof. Jin-Yi Yu Prof. Jin-Yi Yu 1 Vertical Structure of the Atmosphere Composition of the Atmosphere (inside the DRY homosphere) composition temperature electricity Water vapor (0-0.25%) 80km (from Meteorology Today) ESS200 (from The Blue Planet) ESS200 Prof. Jin-Yi Yu Prof. Jin-Yi Yu Origins of the Atmosphere What Happened to H2O? When the Earth was formed 4.6 billion years ago, Earth’s atmosphere was probably mostly hydrogen (H) and helium (He) plus hydrogen The atmosphere can only hold small fraction of the mass of compounds, such as methane (CH4) and ammonia (NH3). -
Unit 1 Atmosphere It Is the Air Blanket Which Surrounds the Planet And
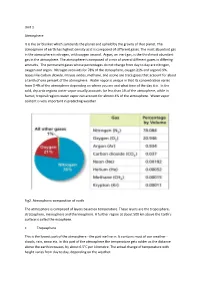
Unit 1 Atmosphere It is the air blanket which surrounds the planet and upheld by the gravity of that planet. The atmosphere of earth has highest density as it is composed of different gases. The most abundant gas in the atmosphere is nitrogen, with oxygen second. Argon, an inert gas, is the third most abundant gas in the atmosphere. The atmosphere is composed of a mix of several different gases in differing amounts. The permanent gases whose percentages do not change from day to day are nitrogen, oxygen and argon. Nitrogen accounts for 78% of the atmosphere, oxygen 21% and argon 0.9%. Gases like carbon dioxide, nitrous oxides, methane, and ozone are trace gases that account for about a tenth of one percent of the atmosphere. Water vapor is unique in that its concentration varies from 0-4% of the atmosphere depending on where you are and what time of the day it is. In the cold, dry artic regions water vapor usually accounts for less than 1% of the atmosphere, while in humid, tropical regions water vapor can account for almost 4% of the atmosphere. Water vapor content is very important in predicting weather. Fig2. Atmospheric composition of earth The atmosphere is comprised of layers based on temperature. These layers are the troposphere, stratosphere, mesosphere and thermosphere. A further region at about 500 km above the Earth's surface is called the exosphere. • Troposphere This is the lowest part of the atmosphere - the part we live in. It contains most of our weather - clouds, rain, snow etc. In this part of the atmosphere the temperature gets colder as the distance above the earth increases, by about 6.5°C per kilometre. -

6Th Grade SDP Science Teachers
Curriculum Guide for 6th Grade SDP Science Teachers Please note: Pennsylvania & Next Generation Science Standards as well as Instructional Resources are found on the SDP Curriculum Engine Prepared by :Emily McGady, Science Curriculum Specialist 8/2017 1 6th Grade (Earth) Science Curriculum Term 1 (9/5-11/13/17) Topic: Landforms Duration: 9-10 Weeks Performance Objectives SWBAT: • analyze models of Earth’s various landforms (e.g., mountains, peninsulas) IOT identify and describe these landforms. • compare and contrast different bodies of water on Earth (e.g., streams, ponds, lakes, creeks) IOT categorize water systems as lentic or lotic. • compare and contrast different water systems (e.g., wetlands, oceans, rivers, watersheds) IOT describe their relationship to each other as well as to landforms. • create a stream table IOT explore relationships between systems, water, and land. • identify features of maps and diagrams IOT interpret what models represent. • describe Earth’s natural processes IOT analyze their effects on the Earth’s systems. • give examples of weathering and erosion IOT describe the impacts of weathering and erosion on landforms. • construct a scientific explanation based on evidence IOT determine how the uneven distributions of Earth’s mineral, energy, and groundwater resources are the result of past and current geoscience processes. • construct an explanation based on evidence IOT determine how geoscience processes have changed Earth’s surface at varying time and spatial scales. Define the criteria and constraints of a design problem IOT provide sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions. -

Evidence of the Complexity of Aerosol Transport in the Lower Troposphere on the Namibian Coast During AEROCLO-Sa
Atmos. Chem. Phys., 19, 14979–15005, 2019 https://doi.org/10.5194/acp-19-14979-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Evidence of the complexity of aerosol transport in the lower troposphere on the Namibian coast during AEROCLO-sA Patrick Chazette1, Cyrille Flamant2, Julien Totems1, Marco Gaetani2,3, Gwendoline Smith1,3, Alexandre Baron1, Xavier Landsheere3, Karine Desboeufs3, Jean-François Doussin3, and Paola Formenti3 1Laboratoire des Sciences du Climat et de l’Environnement (LSCE), Laboratoire mixte CEA-CNRS-UVSQ, UMR CNRS 1572, CEA Saclay, 91191 Gif-sur-Yvette, France 2LATMOS/IPSL, Sorbonne Université, CNRS, UVSQ, Paris, France 3Laboratoire Interuniversitaire des Systèmes Atmosphériques (LISA) UMR CNRS 7583, Université Paris-Est-Créteil, Université de Paris, Institut Pierre Simon Laplace, Créteil, France Correspondence: Patrick Chazette ([email protected]) Received: 29 May 2019 – Discussion started: 3 June 2019 Revised: 27 October 2019 – Accepted: 28 October 2019 – Published: 11 December 2019 Abstract. The evolution of the vertical distribution and opti- ern Africa by the equatorward moving cut-off low originat- cal properties of aerosols in the free troposphere, above stra- ing from within the westerlies. All the observations show a tocumulus, is characterized for the first time over the Namib- very complex mixture of aerosols over the coastal regions of ian coast, a region where uncertainties on aerosol–cloud cou- Namibia that must be taken into account when investigating pling in climate simulations are significant. We show the high aerosol radiative effects above stratocumulus clouds in the variability of atmospheric aerosol composition in the lower southeast Atlantic Ocean.