WP8 1 Determination of Market Potential for Selected Platform Chemicals Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Design and Development of Molecularly Imprinted Polymers and Imprinted Sensors
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Design and development of molecularly imprinted polymers and imprinted sensors Ferdia Bates Doctoral Thesis Doctoral Studies in Chemistry Director: Manel del Valle Zafra Departament de Química Facultat de Ciències 2016 Declaration Thesis submitted to aspire for the doctoral degree Ferdia Bates Director's approval: Dr. Manel de Valle Zafra Professor of Analytical Chemistry Bellaterra (Ceerdanyola del Vallès), September 2016 III Funding acknowledgement This present dissertation has been carried out in the laboratories of the Grup de Sensors i Biosensors of the Department de Química in the Universitat Autònoma de Barcelona, with the support of the Marie Curie Actions fellowship FP7-PEOPLE-2010-ITN- and the financial support of the Ministry of Economy and Innovation (MINECO) project “Electronic tongue fingerprinting: aplicaciones en el campo alimentario y de seguridad” (MCINN, CTQ2013-41577-P). Grup de Senors i Biosensors Unitat de Química Analítica Department de Química Universitat Autónoma de Barcelona Edifici Cn 08193, Bellatera IV Acknowledgments For my Masters supervisor at Cranfield university, Doctor Yi Ge, my respected colleague and friend, I would like to offer my most heart-felt thanks; without Dr Ge's encouragement, advise and reference, I most probably would not have pursued a doctorate as a career choice. -

Itaconic Acid Production by Microorganisms: a Review
Current Research Journal of Biological Sciences 7(2): 37-42, 2015 ISSN: 2041-076X, e-ISSN: 2041-0778 © Maxwell Scientific Organization, 2015 Submitted: September 29, 2014 Accepted: November 26, 2014 Published: April 20, 2015 Itaconic Acid Production by Microorganisms: A Review Helia Hajian and Wan Mohtar Wan Yusoff School of Bioscience and Biotechnology, Faculty of Science and Technologi, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia Abstract: Itaconic acid (C5H6O4) is an organic acid with unique structure and characteristics. In order to promote the bio-based economy, the US-Department of Energy (DOE) assigned a “top-12” of platform chemicals, which include numerous of organic acids. In particular di-carboxylic acids, like itaconic acid, can be used as monomers for bio-polymers. Thus the need to produce itaconic acid attracts much attention. The favored production process is fermentation of carbohydrates by fungi and Aspergillus terreus is the mostly frequently employed commercial producer of itaconic acid. This review reports the current status of use of microorganisms in enhancing productivity. Keywords: Aspergillus, fermentation, itaconic acid, production INTRODUCTION on production of itaconic acid from renewable resources, a drastic shift from the currently prevalent Itaconic acid (methylene succinic acid) is a sourcing from petrochemical feedstock. Asia-Pacific, promising organic acid. It is a white crystalline backed by tremendous impetus from China is poised to unsaturated dicarbonic acid in which one carboxyl emerge as the fastest growing market for itaconic acid group is conjugated to the methylene group. Different at a Compound Annual Growth rate (CAGR) of over microorganisms have been used in industry for the 9.0% through 2017. -

Class 11 Biology Chapter- 13 Respiration in Plants
CLASS 11 BIOLOGY CHAPTER- 13 RESPIRATION IN PLANTS CELLULAR RESPIRATION: The process of conversion of the chemical energy of organic substances into a metabolically usable energy within living cells is called cellular respiration. TYPES OF CELLULAR RESPIRATION: (i) Aerobic respiration: The process of respiration which requires molecular oxygen. (ii) Anaerobic respiration: The process of respiration which does not require molecular oxygen and occurs in the cytoplasm. MECHANISM OF RESPIRATION: Following are the steps- 1. GLYCOLYSIS / EMP Pathway: It involves a series of closely integrated reactions in which hexose sugars(usually glucose) are converted into pyruvic acid. It is common in both aerobic and anaerobic reactions. It occurs in the cytoplasm. It does not require oxygen. Gollowing are the steps of GLYCLOLYSIS: (i) Conversion of glucose to Fructose-1,6-diphosphate: First phosphorylation: Glucose is converted to Glucose -6-phosphate in the presence of enzyme hexokinase and Mg++ ions and energy in the form of ATP. Isomerization: Glucose-6-phosphate is converted to Fructose-6-phosphate in the presence of phosphohexoisomerase. Second phosphorylation: Fructose-6-phosphate is converted to Fructose-1,6- diphosphate by the use of energy in the form of ATP. (ii) Formation of pyruvic acid from fructose -1,6-diphosphate: Cleavage: Fructose-1,6-diphosphate splits into 3-phosphoglyceraldehyde and Dihydroxyacetone phosphate in the presence of enzyme aldolase. Phosphorylation and oxidative dehydrogenase: 3-phosphoglyceraldehyde is converted to 1,3-biphosphoglyceric acid. ATP generation(first): 1,3-biphosphoglyceric acid is converted to 3-phosphoglyceric acid in the presence of Mg++ and phosphoglycerokinase . Isomerization: 3-phosphoglyceric acid is converted to 2-phosphoglyceric acid in the presence of Mg++. -

|||||||III US005457040A United States Patent (19) 11) Patent Number: 5,457,040 Jarry Et Al
|||||||III US005457040A United States Patent (19) 11) Patent Number: 5,457,040 Jarry et al. (45) Date of Patent: Oct. 10, 1995 (54) PRODUCTION OF ITACONIC ACID BY 4,740,464 4/1988 Holdom et al.......................... 435/135 FERMENTATION 5,231,016 7/1993 Cros et al. .............................. 435/142 (75) Inventors: Alain Jarry, Maisonnay; Yolaine FOREIGN PATENT DOCUMENTS Seraudie, Melle, both of France 0697653 11/1964 Canada .................................. 435/142 0341112 11/1989 European Pat. Off. 73) Assignee: Rhone-Poulenc Chimie, Courbevoie, 1327.937 4/1963 France. France 0052990 7/1973 Japan ..................................... 435/142 0507633 3/1976 U.S.S.R. ................ ... 435/142 0602866 6/1948 United Kingdom....... ... 435/142 (21 Appl. No.: 205,646 0795401 5/1958 United Kingdom....... ... 435/142 22 Filed: Mar. 4, 1994 0878152 9/1961 United Kingdom ................... 435/142 Primary Examiner-Herbert J. Lilling (30) Foreign Application Priority Data Attorney, Agent, or Firm-Burns, Doane, Swecker & Mathis Mar. 12, 1993 (FR) France ................................... 93 02844 57) ABSTRACT (51) Int. Cl. ............................................ C12P 7/44 Itaconic acid and/or salt thereof is produced via aerobic 52 U.S. Cl. ................ 435/142; 435/913 microbial fermentation, for example by means of the species 58) Field of Search .......... - - - - - 435/142,913 Aspergillus terreus or Aspergillus itaconicus, of a nutrient medium containing a source of assimilable carbon, such 56) References Cited carbon source at least in part comprising an effective amount U.S. PATENT DOCUMENTS of glycerol. 3,873,425 3/1975 Kobayashi et al. ..................... 435/145 1 Claim, No Drawings 5,457,040 1. 2 PRODUCTION OF TACONCACD BY the other carbon substrates indicated above, glycerol pre FERMENTATION sents the distinct advantage of being especially advanta geous from an economic standpoint. -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -
STAC-V : Chemical Resistance List Max Temperature
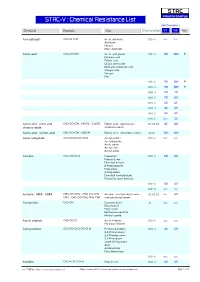
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Methods for Producing Isomers of Muconic Acid
(19) TZZ _Z_T (11) EP 2 521 770 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 9/02 (2006.01) C12N 9/88 (2006.01) 25.11.2015 Bulletin 2015/48 C12N 15/52 (2006.01) C12P 7/44 (2006.01) (21) Application number: 11700591.8 (86) International application number: PCT/US2011/020681 (22) Date of filing: 10.01.2011 (87) International publication number: WO 2011/085311 (14.07.2011 Gazette 2011/28) (54) METHODS FOR PRODUCING ISOMERS OF MUCONIC ACID AND MUCONATE SALTS VERFAHREN ZUR HERSTELLUNG VON ISOMEREN AUS MUCONSÄURE UND MUCONATSALZEN PROCÉDÉS POUR PRODUIRE DES ISOMÈRES D’ACIDE MUCONIQUE ET DE SELS DE MUCONATE (84) Designated Contracting States: US-A- 4 731 328 US-A- 5 616 496 AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • NIU WEI ET AL: "Benzene- freesynthesis of adipic PL PT RO RS SE SI SK SM TR acid", BIOTECHNOLOGY PROGRESS, AMERICAN INSTITUTE OF CHEMICAL (30) Priority: 08.01.2010 US 335638 P ENGINEERS, US, vol. 18, no. 2, 1 March 2002 (2002-03-01), pages201-211, XP002568326, ISSN: (43) Date of publication of application: 8756-7938, DOI: DOI:10.1021/BP010179X 14.11.2012 Bulletin 2012/46 [retrieved on 2002-02-12] • WU C-M ET AL: "Microbial synthesis of cis,cis- (73) Proprietor: Amyris, Inc. muconic acid by Sphingobacterium sp. GCG Emeryville, CA 94608 (US) generated from effluent of a styrene monomer (SM) production plant", ENZYME AND (72) Inventors: MICROBIAL TECHNOLOGY, STONEHAM, MA, • BUI, Vu US,vol. -

Citric Acid and Itaconic Acid Accumulation: Variations of the Same Story?
Applied Microbiology and Biotechnology https://doi.org/10.1007/s00253-018-09607-9 MINI-REVIEW Citric acid and itaconic acid accumulation: variations of the same story? Levente Karaffa 1 & Christian P. Kubicek2,3 Received: 5 December 2018 /Revised: 28 December 2018 /Accepted: 28 December 2018 # The Author(s) 2019 Abstract Citric acid production by Aspergillus niger and itaconic acid production by Aspergillus terreus are two major examples of technical scale fungal fermentations based on metabolic overflow of primary metabolism. Both organic acids are formed by the same metabolic pathway, but whereas citric acid is the end product in A. niger, A. terreus performs two additional enzymatic steps leading to itaconic acid. Despite of this high similarity, the optimization of the production process and the mechanism and regulation of overflow of these two acids has mostly been investigated independently, thereby ignoring respective knowledge from the other. In this review, we will highlight where the similarities and the real differences of these two processes occur, which involves various aspects of medium composition, metabolic regulation and compartmentation, transcriptional regulation, and gene evolution. These comparative data may facilitate further investigations of citric acid and itaconic acid accumulation and may contribute to improvements in their industrial production. Keywords Aspergillus niger . Aspergillus terreus . Citric acid . Itaconic acid . Submerged fermentation . Overflow metabolism Introduction terreus—was patented in the next decade (Kane et al. 1945). Before World War II, organic acid manufacturing was exclu- Citric acid (2-hydroxy-propane-1,2,3-tricarboxylic acid) sively performed by the labor-intensive and relatively low- and itaconic acid (2-methylene-succinic acid or 2- yield surface method (Doelger and Prescott 1934;Calam methylidenebutanedioic acid) are the most well-known exam- et al. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Antibiotics As a Feed Additive Promote Growth of Chickens by Modulating the Gut Microbiome
Antibiotics as a Feed Additive Promote Growth of Chickens by Modulating the Gut Microbiome Xiangkai Li ( [email protected] ) Lanzhou University https://orcid.org/0000-0002-5704-9791 Ying Wu Lanzhou University Liang Peng Lanzhou University Rong Han Lanzhou University pengya Feng Lanzhou University Aman Khan Lanzhou University Pu Liu Lanzhou University Research Keywords: Antibiotic, Gut microbiota, Metabonomic, Lipid and amid acid metabolism, Immune response Posted Date: June 7th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-571302/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/31 Abstract Background: Antibiotics widely used as growth promoters in the agricultural industry, but their mechanisms have not been fully explored. Antibiotics has a connection effect on the gut microbiome which plays a vital role in host metabolism and immune response. Here, we investigated the association of antibiotic and gut microbiome in broiler chicken. Methods: Polymyxin (PMX) and amoxicillin (AMX) were selected as feed additives in broiler chicken, and gut bacterial composition assessed by quantitative real-time PCR and high-throughput sequencing of bacterial 16S rRNA. Metabolome and lipidomic of feces and serum samples were analyzed using liquid chromatograpy-tandem mass spectrometry (LC-MS). We further assessed changes in the microbiota and metabolism which underwent antibiotics treatment. Results: The administration of antibiotic increasing, the average weight of chickens by up to 4.9% and altered number and structure of the intestinal microora compared to the un-treated group. The bacterial component of gut microbiota in antibiotic groups was showed a lower prevalence of Firmicutes and Bacteroidetes phyla and higher prevalence of a high diversity (Proteobacteria). -

Citric Acid Cycle
Lecture: 4 Biochemistry Anwar J Almzaiel Citric acid cycle Citric acid cycle (Krebs cycle, tricarboxylic acid cycle) is a series of reactions in mitochondria that bring about the catabolism of acetyl residues, liberating hydrogen equivalents, which upon oxidation lead to the release of most the energy of tissues fuels. The major function of cycle is to act as the final common pathway for oxidation of fatty acids, carbohydrates and proteins. It includes the electron transport system, the energy is produced in large amounts. Several of these processes are carried out in many tissues but the liver is the only tissue in which all occur to a significant extent, thus it is lethal when large numbers of hepatic cell are damaged or replaced by connective tissue, as in acute hepatitis and cirrhosis respectively. This cycle is found in the mitochondria. It starts with pyruvic acid which comes from cytoplasm to the mitochondria to be converted with coenzymes (NAD) and enzymes into acetyl COA + Pyruvic acid + COA +NAD Acetyl CoA +NADH+ CO2 The enzymes needed are found in the mitochondria close to the enzymes of the respiratory chain The oxidation of pyruvate to acetyl-CoA Before pyruvate can enter the citric acid cycle, it must be transported into the mitochondria via a special pyruvate transporter that aids its passage across the inner mitochondrial membrane. Within the mitochondria, pyruvate is oxdatively decarboxylated into acetyl COA. The conversion of pyruvic acid to acetyl COA involves 5 types of reaction and each reaction is catalysed by different enzyme system these enzymes act as a multi enzyme system (complex). -

I Norgan Ic C He Mi Str Y
View Article Online / Journal Homepage / Table of Contents for this issue INORGANIC CHEMISTRY. 443 I n o r g a n ic C h e mi s t r y. Composition of Atmospheres which Extinguish Flame. By FRANKCLOWES ( PTOC. Roy. Soc., 1894, 56, 2-6) .--The experimental flame burning at a platinum jet 1 mm. in diameter, was 0.75 in. in height; it was gradually lowered into a cylinder containing the atmosphere of mixed gases, and these were considered to be in extinctive proportions if the flame was extinguished during its downward passage, or immediately on attaining its lowest position in the cylinder. The gaseous mixture was regarded as containing the minimum quantitr of extinctive gas, when the flame on being lowered into another mixture containing 1 per cent. less of such gas continued to burn in it for a few seconds before being ex- tinguished. Experiments made with flames of hydrogen and Published on 01 January 1895. Downloaded 25/10/2014 07:12:09. alcohol, varying from 0.4 in. to 1.5 in. in height, show that the varying dimensions of the flame are without influence on the proportion of carbonic anhydride in the air necessary to pro- duce extinction. Characteristic differences were observed between the behaviour of wick-fed flames and that of gas-fed flames when they were introduced into an atmosphere which extinguished them, the wick-fed flames gradually diminishing in size until they vanished, whilst the gas-fed flames gradually increased in size, becoming paler and apparently lower in temperature until they suddenly expired.