Shale Gas and Groundwater Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Sodium Diacetate
Technical Data Sheet Ref.: 2019_12v09 Sodium Diacetate Stability Description SodiumDiacetate is stable for 3 years from Sodium Diacetate is available as a free date of production. Physical stability and flowing white crystal, containing appearance may change before the end of approximately equimolar amounts of shelf-life if not stored single-stacked in Sodium Acetate and acetic acid. closed original packaging, dry and at room temperature. PRODUCT PROPERTIES Handling Product is classified as irritant. Always Product name Sodium Diacetate check the Safety Data Sheet and label Formula CH3COONaCH3COOH before using the product. Molecular weight 142.08 g/mol CAS No. 126-96-5 Packaging EINECS No. 2048149 Sodium Diacetate crystal is packed in 900 kg big bags. HS code US 2915.29.5000 HS code EU 2915.29.00 Safety precautions Please see the Safety Data Sheet before handling the material. Product grades Sodium Diacetate has a free acetic acid content of 40% - 43%. Application Sodium Diacetate is used in haemodialysis. The product is also used in other applications where it is advantageous to use a solid source of free acetic acid, e.g. for safer handling and reduced transportation costs. Warranty. This information herein is offered as a guide and is believed to be accurate and reliable as of the date of the printing. The values given are not to be considered as a warranty and they are subject to change without prior notice. For additional information regarding our products or for information concerning current specifications, please contact our Technical Service. Niacet Corporation www.niacet.com Niacet b.v. 400, 47th Street P.O. -

Meat Curing and Sodium Nitrite
MEDIA MYTHCRUSHER Meat Curing and Sodium Nitrite The use of nitrite to produce cured meats like salami, ham, bacon and hot dogs, is a safe, regulated practice that has distinct public health benefits. However, much confusion and even mythology surrounds nitrite. Being mindful of key words and statistics and providing appropriate context can help reporters improve the accuracy of their coverage and the information that is passed on to readers and viewers. We’ve compiled ten tips to improve accuracy when writing about the use of sodium nitrite in cured meats. #1: Nitrite is not ‘unnatural’. Before the terms nitrate and nitrite are often used refrigeration was available, humans salted and interchangeably, meat companies mainly use dried meat to preserve it. It was discovered sodium nitrite to cure meat, not sodium nitrate. that the nitrate in saltpeter was extremely At the turn of the 20th century, German effective in causing a chemical reaction known scientists discovered nitrite (and not nitrate) as “curing.” Not only did this give meat a was the active form of these curing salts. When distinct taste and flavor, it also preserved it and added directly, rather than as nitrate, meat prevented the growth of Clostridium botulinum, processors can have better control of this which causes botulism. important curing ingredient and more closely manage how much they are adding. Later on, scientists came to understand that nitrate naturally found in the environment #3: Cured meats are a miniscule source of converts to nitrite when in the presence of total human nitrite intake. Scientists say that certain bacteria. -

Effects of Sodium Citrate Plus Sodium Diacetate and Buffered Vinegar on Escherichia Coli O157:H7 and Psychrotrophic Bacteria in Brine-Injected Beef
359 Journal of Food Protection, Vol. 74, No. 3, 2011, Pages 359–364 doi:10.4315/0362-028X.JFP-10-294 Copyright G, International Association for Food Protection Effects of Sodium Citrate plus Sodium Diacetate and Buffered Vinegar on Escherichia coli O157:H7 and Psychrotrophic Bacteria in Brine-Injected Beef AMUDHAN PONRAJAN,1,2 MARK A. HARRISON,1 JACOB R. SEGERS,2 BRADLEY K. LOWE,2 RUSSELL O. MCKEITH,2 T. DEAN PRINGLE,2 KARINA G. MARTINO,1 JAKE H. MULLIGAN,1 AND ALEXANDER M. STELZLENI2* Downloaded from http://meridian.allenpress.com/jfp/article-pdf/74/3/359/1685103/0362-028x_jfp-10-294.pdf by guest on 01 October 2021 1Department of Food Science and Technology and 2Department of Animal and Dairy Sciences, University of Georgia, Athens, Georgia 30602, USA MS 10-294: Received 15 July 2010/Accepted 4 December 2010 ABSTRACT The objective of this research was to examine the effects of sodium citrate plus sodium diacetate or buffered vinegar on Escherichia coli O157:H7 and psychrotrophic bacteria when incorporated in brine solutions for injected beef. Two experiments were conducted in which 30 top rounds and 30 top sirloins were injected (110%) to contain (i) 0.5% sodium chloride and 0.4% sodium tripolyphosphate as the control (CNT); (ii) CNT with a 1% solution of 80% sodium citrate plus 20% sodium diacetate (SCzD); or (iii) CNT with 2% buffered vinegar (VIN) in the final product. For the E. coli challenge, muscles were surface inoculated to target 6 log CFU/cm2. After injection and 10 days of storage in a vacuum package (4uC), one half of each muscle was sampled raw and the other half was cooked to an internal temperature of 60uC with a 12-min hold. -
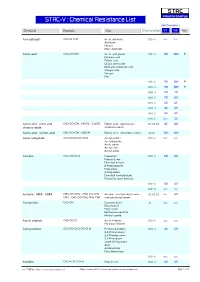
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium Perfringens Spores in Ham During Abusive Cooling
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations, Theses, & Student Research in Food Science and Technology Food Science and Technology Department Fall 9-19-2011 Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium perfringens Spores in Ham during Abusive Cooling Mauricio A. Redondo University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/foodscidiss Part of the Food Chemistry Commons, Food Microbiology Commons, and the Food Processing Commons Redondo, Mauricio A., "Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium perfringens Spores in Ham during Abusive Cooling" (2011). Dissertations, Theses, & Student Research in Food Science and Technology. 18. https://digitalcommons.unl.edu/foodscidiss/18 This Article is brought to you for free and open access by the Food Science and Technology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations, Theses, & Student Research in Food Science and Technology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. EFFECT OF SODIUM NITRITE, SODIUM ERYTHORBATE AND ORGANIC ACID SALTS ON GERMINATION AND OUTGROWTH OF CLOSTRIDIUM PERFRINGENS SPORES IN HAM DURING ABUSIVE COOLING By Mauricio Redondo-Solano A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Food Science and Technology Under the supervision of Professor Harshavardhan Thippareddi Lincoln, Nebraska September, 2011 EFFECT OF SODIUM NITRITE, SODIUM ERYTHORBATE AND ORGANIC ACID SALTS ON GERMINATION AND OUTGROWTH OF CLOSTRIDIUM PERFRINGENS SPORES IN HAM DURING ABUSIVE COOLING Mauricio Redondo-Solano, M. -

Summary of Fracfocus 1.0 Hydraulic Fracturing Data User Guide for State Summaries
Summary of FracFocus 1.0 Hydraulic Fracturing Data User Guide for State Summaries The Ground Water Protection Council (GWPC) and the Interstate Oil and Gas Compact Commission (IOGCC) launched the FracFocus Chemical Disclosure Registry in 2011 to provide information to the public about the chemicals used in hydraulic fracturing fluids. Background on FracFocus 1.0 Operators submit information on the composition of fluids used for hydraulic fracturing operations at individual oil and gas production wells on specific dates. These submissions, or "disclosures" as they are commonly called, are searchable by the public and available as PDF files. Disclosures provide information on the fracture date, location, well number and name, well operator, intended production type of the well, depth, total water volume used, trade name and purpose of additives, and ingredients in fracturing fluid, as shown below. Operators initially disclosed information to FracFocus 1.0 voluntarily. During the period of time studied in this report (January 1, 2011 through February 28, 2013), 14 states enacted regulations requiring oil and gas production well operators to disclose hydraulic fracturing chemicals to the public. Four of these states require reporting to FracFocus (one allows reporting to the state, which will then submit the information to FracFocus), two states require reporting to both FracFocus and the state, three states offer the choice of reporting to the state or FracFocus, and five states require reporting directly to the state. Example disclosure from FracFocus 1 Summary of FracFocus 1.0 Hydraulic Fracturing Data User Guide for State Summaries How the EPA Conducted Its Analysis of FracFocus 1.0 Data The goal of this project was to gain a better understanding of the composition of fracturing fluid and water usage for hydraulic fracturing. -

I Norgan Ic C He Mi Str Y
View Article Online / Journal Homepage / Table of Contents for this issue INORGANIC CHEMISTRY. 443 I n o r g a n ic C h e mi s t r y. Composition of Atmospheres which Extinguish Flame. By FRANKCLOWES ( PTOC. Roy. Soc., 1894, 56, 2-6) .--The experimental flame burning at a platinum jet 1 mm. in diameter, was 0.75 in. in height; it was gradually lowered into a cylinder containing the atmosphere of mixed gases, and these were considered to be in extinctive proportions if the flame was extinguished during its downward passage, or immediately on attaining its lowest position in the cylinder. The gaseous mixture was regarded as containing the minimum quantitr of extinctive gas, when the flame on being lowered into another mixture containing 1 per cent. less of such gas continued to burn in it for a few seconds before being ex- tinguished. Experiments made with flames of hydrogen and Published on 01 January 1895. Downloaded 25/10/2014 07:12:09. alcohol, varying from 0.4 in. to 1.5 in. in height, show that the varying dimensions of the flame are without influence on the proportion of carbonic anhydride in the air necessary to pro- duce extinction. Characteristic differences were observed between the behaviour of wick-fed flames and that of gas-fed flames when they were introduced into an atmosphere which extinguished them, the wick-fed flames gradually diminishing in size until they vanished, whilst the gas-fed flames gradually increased in size, becoming paler and apparently lower in temperature until they suddenly expired. -

The Iron-Dependent Cyanide and Hydrogen Peroxide Co-Toxicity in Escherichia Coli and Its Catastrophic Consequences for the Chromosome
THE IRON-DEPENDENT CYANIDE AND HYDROGEN PEROXIDE CO-TOXICITY IN ESCHERICHIA COLI AND ITS CATASTROPHIC CONSEQUENCES FOR THE CHROMOSOME BY TULIP MAHASETH DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Microbiology in the Graduate College of the University of Illinois at Urbana-Champaign, 2015 Urbana, Illinois Doctoral Committee: Professor Andrei Kuzminov, Chair and Director of Research Professor John E. Cronan Professor Jeffrey F. Gardner Associate Professor Carin K. Vanderpool ABSTRACT 2+ Hydrogen peroxide (H2O2) can oxidize cytoplasmic ferrous ions (Fe ) to produce highly reactive hydroxyl radicals (•OH) via Fenton’s reaction that can damage various biomolecules causing oxidative stress. Even though at concentrations higher than 20 mM H2O2 by itself can efficiently kill micro-organisms, it is metabolically impossible for eukaryotic cells to generate H2O2, an uncharged molecule, in such large quantities inside the cell. We propose that potentiation of physiologically relevant amounts of H2O2 by various small molecules serves as a more feasible and safe mechanism to combat invading microbes. NO potentiation of H2O2 toxicity is a known bactericidal weapon employed by macrophages. In fact, in human neutrophils activated by bacterial infection, the myeloperoxidase enzyme catalyzes the formation of hydrogen cyanide (HCN) from serum thiocyanate (SCN-). In the past, researchers have reported that a combination of low millimolar doses of H2O2 and cyanide (CN), which are individually bacteriostatic, caused rapid synergistic killing in Escherichia coli. Our aim is to understand the immune cells antimicrobial responses by investigating the mechanism of CN potentiation of H2O2 toxicity and its chromosomal consequences. We have found that the ability of CN to recruit iron from intracellular depots such as ferritin contributes to its potentiation of H2O2 toxicity, whereas the major stationary phase intracellular iron depot protein, Dps, can sequester this iron, thereby quelling Fenton's reaction. -

Bioorganic Chemistry
Hermann Dugas Christopher Penney Bioorganic Chemistry A Chemical Approach to Enzyme Action With 82 Figures Spri nger-Verlag New York Heidelberg Berlin Dr. Hermann Dugas Dr. Christopher Penney Departement de Chimie Connaught Research Institute Universite de Montreal Willowdale, Ontario Montreal, Quebec Canada M2N 5T8 Canada H3C 3Vl Series Editor: Prof. Charles R. Cantor Columbia University Box 608 Havemeyer Hall New York, New York 10027 USA Cover: The green illustration represents the hypothetical mode of binding of a rigid structural analogue of N-benzoyl-L-phenylalanine methyl ester at the active site of a-chymotrypsin. The illustration emphasizes the equilibration toward the favored configuration (see text page 224). The background design is taken from a diagrammatic representation of the primary structure of a-chymotrypsin. After Nature with permission [B.W. Matthews, P.B. Sigler, R. Henderson, and D.M. Blow (1967), Nature 214, 652-656]. Library of Congress Cataloging in Publication Data Dugas, Hermann, 1942- Bioorganic chemistry. (Springer advanced texts in chemistry.) Bibliography: p. Includes index. 1. Enzymes. 2. Biological chemistry. 3. Chemistry,. Organic. I. Penney, Christopher, 1950- joint author. II. Title. m. Series. [DNLM: 1. Biochemistry. 2. Enzymes-Metabolism. QUl35 D866b] QP60 1. D78 574.19'25 80-16222 All rights reserved. No part of this book may be translated or reproduced in any form without written permission from Springer-Verlag. The use of general descriptive names, trade names, trademarks, etc. in this publication, even if the former are not especially identified, is not to be taken as a sign that such names, as understood by the Trade Marks and Merchandise Marks Act, may accordingly be used freely by anyone.