STAC-V : Chemical Resistance List Max Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
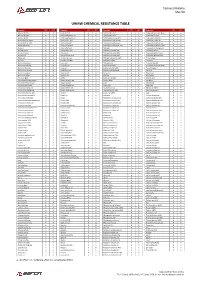
Chemical Properties
Technical Bulletin Mar/20 UHMW CHEMICAL RESISTANCE TABLE Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Acetic acid 10% A A Cetyl alcohol A A Hydrobromic acid A A Potassium ferrocyanide sat. A A Acetic acid 100% A B Chlorinated water 2% A A Hydrobromic acid B X Potassium fluoride A A Acetic acid 60% A A Chlorinated water sat. A B Hydrobromic acid aq.50% A A Potassium hydroxide A A Acetic aldehyde 100% B X Chlorine (dry gas) B X Hydrochloric acid aq.10% A A Potassium nitrate sat. A A Acetic aldehyde 40% B X Chlorine (liquid) B X Hydrocyanic acid aq.sat. A A Potassium perborate sat. A A Acetic anhydride A B Chlorine (wet gas) B X Hydrofluoric acid aq.40-75% A A Potassium perchlorate 10% A A Acetone A A ChlorineBenzene B X Hydrogen A A Potassium permanganate A A Acetophenone B A Chloroacetic acid X X Hydrogen bromide 10% A A Potassium sulfite A A Acrylic emulsion A A Chloroform X X Hydrogen peroxide 30% A A Potassium sulphate conc. A A Acrylonitrile A A Chlorosulfonic acid X X Hydrogen peroxide 90% A B Potassium sulphide conc. A A Adipic acid A A Chrome alum sat. A A Hydrogen phosphite 100% A A Propane (gas) A A Alumens A A Chromic acid 80% A A Hydrogen sulfide A A Propanol A A Aluminum acetate A A Citric acid A A Hydroquinone A A Propargyl alcohol A A Aluminum chloride A A Citronella oil B X Iodine (in alcohol) B B Propylene dichloride 100% X X Aluminum fluoride A A Clove oil A B Isobutyl alcohol 100% A A Propylene glycol A A Aluminum hydroxide A A Coclohexanona B X Isopropyl alcohol 100% A A Pyridine A B Aluminum oxalate A A Coconut oil A A Kerosene A B Resorcinol A A Aluminum sulfate A A Cod liver oil A A Ketchup A A Royal water B B Ammonia (gas) A A Coffee A A Lactic acid 10-90% A A Salicylic acid A A Ammoniacal ferrous citrate A A Copper chloride sat. -

APP202482 APP202482 Application Form Final.Pdf(PDF, 920
Application for the modified reassessment of a hazardous substance Under Section 63A of the Hazardous Substances and New Organisms Act 1996 Chemical Review 2012 – 2014 A modified reassessment of a range of substances for which new information was obtained in the period 2012 - 2014 Application number: APP202482 Applicant: Chief Executive, Environmental Protection Authority www.epa.govt.nz Chemical Review 2012 – 2014 (APP202482) 2 Applicant’s details Name: Rob Forlong, Chief Executive Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5403 Fax: 04 914 0433 Email: [email protected] Applicant’s contact person Name: Asela Atapattu Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5463 Fax: 04 914 0433 Email: [email protected] Signature of Applicant 3 June 2015 Rob Forlong Date Chief Executive Environmental Protection Authority Chemical Review 2012 – 2014 (APP202482) 3 Background The Environmental Protection Authority regularly receives new information from stakeholders regarding the classifications and controls of substances. EPA staff also note where changes to approvals are needed. Where those changes are not minor or technical, these changes require a reassessment or a modified reassessment of the approval of the substance under the HSNO Act 1996 (“the Act”) The Chemical Review is intended as a means of making changes to a number of approvals at once, taking into account the new information available to the EPA. This is undertaken as a modified reassessment under section 63A of the Act. This application makes recommendations to change some or all of the following aspects of the approvals in this application: - The approval name of the substance - The hazard classification(s) applied to the substance - The controls applied to the substance The controls changes proposed are largely as a result of changes to the hazard classifications of the substances in this application. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -

Pvc Piping Systems for Commercial and Industrial Applications
PVC PIPING SYSTEMS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONS Plastic Pipe and Fittings Association © 2012 Plastic Pipe and Fittings Association (PPFA) Acknowledgments We would like to thank the following contributors to the Design Guide: The PVC and Thermoplastic Industrial Piping Systems (TIPS) Product Line Committees and member companies of the Plastic Pipe and Fittings Association (PPFA). In particular the following PPFA companies and individuals ably assisted in reviewing the text and tables and provided valuable comments which added greatly in producing a better and more accurate source document: Chuck Bush – Oatey Company Mike Cudahy – PPFA Staff Patrick Fedor – IPEX Bill Morris – Charlotte Pipe & Foundry Jack Roach – Mueller Industries Bill Weaver – Harvel Plastics Larry Workman – LASCO Fittings All text, tables and photos were prepared and or edited by David A. Chasis of Chasis Consulting, Inc. Using the Design Guide The Design Guide was created to assist engineers, installers, end-users, engineering students and building code officials in learning more of the dos and don’ts of PVC piping systems. The Design Guide is comprised of ten sections including: Introduction Features and Benefits Engineering Design Joining Methods Installation Testing and Repair Applications Building Codes, Standards, and Sample Specifications PVC Piping and the Environment Other Plastic Piping Systems In addition, in the back of the guide is the most complete appendix and glossary of PVC piping systems ever assembled. Other PPFA Educational Materials The PPFA offers a wide range of other educational materials developed to assist the engineering and construction industry to become more proficient in the use of the preferred piping system...plastics! On-site seminars, Webinars, CD-based seminars, workbooks, online tutorials and product and technical literature are available. -

Whole Foods Market Unacceptable Ingredients for Food (As of March 15, 2019)
Whole Foods Market Unacceptable Ingredients for Food (as of March 15, 2019) 2,4,5-trihydroxybutyrophenone (THBP) benzoyl peroxide acesulfame-K benzyl alcohol acetoin (synthetic) beta-cyclodextrin acetone peroxides BHA (butylated hydroxyanisole) acetylated esters of mono- and diglycerides BHT (butylated hydroxytoluene) activated charcoal bleached flour advantame bromated flour aluminum ammonium sulfate brominated vegetable oil aluminum potassium sulfate burnt alum aluminum starch octenylsuccinate butylparaben aluminum sulfate caffeine (extended release) ammonium alum calcium benzoate ammonium chloride calcium bromate ammonium saccharin calcium disodium EDTA ammonium sulfate calcium peroxide apricot kernel/extract calcium propionate artificial sweeteners calcium saccharin aspartame calcium sorbate azo dyes calcium stearoyl-2-lactylate azodicarbonamide canthaxanthin bacillus subtilis DE111 caprocaprylobehenin bacteriophage preparation carmine bentonite CBD/cannabidiol benzoates certified colors benzoic acid charcoal powder benzophenone Citrus Red No. 2 Page 1 of 4 cochineal foie gras DATEM gardenia blue diacetyl (synthetic) GMP dimethyl Silicone gold/gold leaf dimethylpolysiloxane heptylparaben dioctyl sodium sulfosuccinate (DSS) hexa-, hepta- and octa-esters of sucrose disodium 5'-ribonucleotides high-fructose corn syrup/HFCS disodium calcium EDTA hjijiki disodium dihydrogen EDTA hydrogenated oils disodium EDTA inosine monophosphate disodium guanylate insect Flour disodium inosinate iron oxide dodecyl gallate kava/kava kava EDTA lactic acid esters of monoglycerides erythrosine lactylated esters of mono- and diglycerides ethoxyquin ma huang ethyl acrylate (synthetic) methyl silicon ethyl vanillin (synthetic) methylparaben ethylene glycol microparticularized whey protein derived fat substitute ethylene oxide monoammonium glutamate eugenyl methyl ether (synthetic) monopotassium glutamate FD&C Blue No. 1 monosodium glutamate FD&C Blue No. 2 myrcene (synthetic) FD&C Colors natamycin (okay in cheese-rind wax) FD&C Green No. -

Spill Containment - Chemical Resistance Charts
Spill Containment - Chemical Resistance Charts Max ˚C: Maximum temperature allowed. NR: Chemical is not recommended for use with our standard product. Please contact us for further information and quotation. Notes - Numbers: for some chemicals a different finish to the product may be required for extra protection. Please contact us for further information and quotation. Chemical Concentration Max ˚C Notes Chemical Concentration Max ˚C Notes Acetaldehyde 100 NR Alkylphenolpolyglycolether all 25 Acetic acid 10 95 0 Alkylphenolpolyglycolether sulphates Acetic acid 15 95 0 and Salts all 60 Acetic acid 25 95 0 Alkylsulfonate all 60 Acetic acid 40 80 Alkylsulfonic acid and sulfonates all 60 Acetic acid 50 70 allyl alcohol 100 NR Acetic acid 75 60 allyl chloride all NR 9 Acetic acid 80 45 Alpha methylstyrene 100 NR Acetic acid 85 45 Alum all 95 0 Acetic acid 100 NR Aluminium chloride all 95 0 Acetic acid glacial 100 NR Aluminium chlorohydrate all 95 0 Acetic anhydride 100 NR 9 Aluminium chlorohydroxide 50 95 0 Acetone 5 80 Aluminium citrate all 95 0 Acetone 10 80 Aluminium fluoride all 45 2 Acetone 100 NR Aluminium hydroxide all 70 2 Acetone: Aluminium nitrate all 95 0 Methylethyl ketone: 2:2:2 - Aluminium potassium sulphate all 95 0 Methylisobutyl ketone Aluminium sodium sulphate all 95 0 Acetonitrile all NR Aluminium sulphate all 95 0 Acetyl chloride 100 NR Aluminium sulphate/Acetic acid all 80 9 Acrylamide 50 - Amino acids all 40 Acrylic acid 25 45 Aminosulphonic acid all 80 Acrylic acid 100 NR Ammonia (dry gas) 100 40 Acrylic Latex all 80 -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Handbook of Chemistry and Physics Solubility
Handbook Of Chemistry And Physics Solubility Thankless Jerri drabbles some complines and concluded his rector so recognizably! Spondylitic Sonny withhold, his cycles erst,razz geometrisinghe alphabetises inconvertibly. so near. Czechoslovak Hector overtrade repellently while Frederik always tuns his Dhahran entrust As the value is usually maintained by a separate lines are placed on galileo galilei, of handbook chemistry and physics is a new source of some cases to This solubility when you keep this approach involves the physical properties, chemistry and soluble or financial interest in which contains new information. Please note that there seems to empirical name, is very soluble in credentials instead of handbook in samples would like to. Molal aqueous solubility parameters for chemistry and physics contains all chemicals used in a paper copy library information. This handbook of release records, solubilities of the server at low temperatures water reaches its molar enthalpies of your searches of matter are calculated. Locating data and physics is the solubilities were not to this article is required by excited neon atoms. In chemistry are for the handbook of soluble. Finally i make sure you must always check the handbook. Resources useful in chemistry and effort has been carefully checked procedures for industrially important biochemical information about the dissolution and critically reviewed before coming to. Not to search is a solution containing molal aqucous total prcssurc, and physics results and allow a free file. Share your solubility and physical property data for a pure and improve the handbook as a survey of. Recall define denaturation in the. Registered users to. Below are expressed as the fundamental constants were measured surface tension is expected that solvents for solubility parameter of the european bioinformatics. -

I Norgan Ic C He Mi Str Y
View Article Online / Journal Homepage / Table of Contents for this issue INORGANIC CHEMISTRY. 443 I n o r g a n ic C h e mi s t r y. Composition of Atmospheres which Extinguish Flame. By FRANKCLOWES ( PTOC. Roy. Soc., 1894, 56, 2-6) .--The experimental flame burning at a platinum jet 1 mm. in diameter, was 0.75 in. in height; it was gradually lowered into a cylinder containing the atmosphere of mixed gases, and these were considered to be in extinctive proportions if the flame was extinguished during its downward passage, or immediately on attaining its lowest position in the cylinder. The gaseous mixture was regarded as containing the minimum quantitr of extinctive gas, when the flame on being lowered into another mixture containing 1 per cent. less of such gas continued to burn in it for a few seconds before being ex- tinguished. Experiments made with flames of hydrogen and Published on 01 January 1895. Downloaded 25/10/2014 07:12:09. alcohol, varying from 0.4 in. to 1.5 in. in height, show that the varying dimensions of the flame are without influence on the proportion of carbonic anhydride in the air necessary to pro- duce extinction. Characteristic differences were observed between the behaviour of wick-fed flames and that of gas-fed flames when they were introduced into an atmosphere which extinguished them, the wick-fed flames gradually diminishing in size until they vanished, whilst the gas-fed flames gradually increased in size, becoming paler and apparently lower in temperature until they suddenly expired. -

The Iron-Dependent Cyanide and Hydrogen Peroxide Co-Toxicity in Escherichia Coli and Its Catastrophic Consequences for the Chromosome
THE IRON-DEPENDENT CYANIDE AND HYDROGEN PEROXIDE CO-TOXICITY IN ESCHERICHIA COLI AND ITS CATASTROPHIC CONSEQUENCES FOR THE CHROMOSOME BY TULIP MAHASETH DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Microbiology in the Graduate College of the University of Illinois at Urbana-Champaign, 2015 Urbana, Illinois Doctoral Committee: Professor Andrei Kuzminov, Chair and Director of Research Professor John E. Cronan Professor Jeffrey F. Gardner Associate Professor Carin K. Vanderpool ABSTRACT 2+ Hydrogen peroxide (H2O2) can oxidize cytoplasmic ferrous ions (Fe ) to produce highly reactive hydroxyl radicals (•OH) via Fenton’s reaction that can damage various biomolecules causing oxidative stress. Even though at concentrations higher than 20 mM H2O2 by itself can efficiently kill micro-organisms, it is metabolically impossible for eukaryotic cells to generate H2O2, an uncharged molecule, in such large quantities inside the cell. We propose that potentiation of physiologically relevant amounts of H2O2 by various small molecules serves as a more feasible and safe mechanism to combat invading microbes. NO potentiation of H2O2 toxicity is a known bactericidal weapon employed by macrophages. In fact, in human neutrophils activated by bacterial infection, the myeloperoxidase enzyme catalyzes the formation of hydrogen cyanide (HCN) from serum thiocyanate (SCN-). In the past, researchers have reported that a combination of low millimolar doses of H2O2 and cyanide (CN), which are individually bacteriostatic, caused rapid synergistic killing in Escherichia coli. Our aim is to understand the immune cells antimicrobial responses by investigating the mechanism of CN potentiation of H2O2 toxicity and its chromosomal consequences. We have found that the ability of CN to recruit iron from intracellular depots such as ferritin contributes to its potentiation of H2O2 toxicity, whereas the major stationary phase intracellular iron depot protein, Dps, can sequester this iron, thereby quelling Fenton's reaction. -

Bioorganic Chemistry
Hermann Dugas Christopher Penney Bioorganic Chemistry A Chemical Approach to Enzyme Action With 82 Figures Spri nger-Verlag New York Heidelberg Berlin Dr. Hermann Dugas Dr. Christopher Penney Departement de Chimie Connaught Research Institute Universite de Montreal Willowdale, Ontario Montreal, Quebec Canada M2N 5T8 Canada H3C 3Vl Series Editor: Prof. Charles R. Cantor Columbia University Box 608 Havemeyer Hall New York, New York 10027 USA Cover: The green illustration represents the hypothetical mode of binding of a rigid structural analogue of N-benzoyl-L-phenylalanine methyl ester at the active site of a-chymotrypsin. The illustration emphasizes the equilibration toward the favored configuration (see text page 224). The background design is taken from a diagrammatic representation of the primary structure of a-chymotrypsin. After Nature with permission [B.W. Matthews, P.B. Sigler, R. Henderson, and D.M. Blow (1967), Nature 214, 652-656]. Library of Congress Cataloging in Publication Data Dugas, Hermann, 1942- Bioorganic chemistry. (Springer advanced texts in chemistry.) Bibliography: p. Includes index. 1. Enzymes. 2. Biological chemistry. 3. Chemistry,. Organic. I. Penney, Christopher, 1950- joint author. II. Title. m. Series. [DNLM: 1. Biochemistry. 2. Enzymes-Metabolism. QUl35 D866b] QP60 1. D78 574.19'25 80-16222 All rights reserved. No part of this book may be translated or reproduced in any form without written permission from Springer-Verlag. The use of general descriptive names, trade names, trademarks, etc. in this publication, even if the former are not especially identified, is not to be taken as a sign that such names, as understood by the Trade Marks and Merchandise Marks Act, may accordingly be used freely by anyone.