EPDM & FKM Chemical Resistance Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Restriction of Per- and Polyfluoroalkyl Substances (PFAS) Under REACH
Webinar: Restriction of per- and polyfluoroalkyl substances (PFAS) under REACH Questions and answers ECHA organised a webinar on 29 October 2020 on Restriction of per- and polyfluoroalkyl substances (PFAS) under REACH. It explained the REACH restriction process and the status of ongoing work by the Netherlands, Germany, Denmark, Sweden and Norway on a potential restriction to limit the risks to the environment and human health from the manufacture and use of per- and polyfluoroalkyl substances (PFAS). This document compiles the questions and answers from the webinar. Editorial changes have been made to improve clarity, correct spelling mistakes etc. Similar questions have been combined. The document will not be updated. For the most up-to-date advice on PFAS, contact us or refer to our support material. Disclaimer: The European Chemicals Agency and the authorities from the five countries does not accept any liability with regard to the use that may be made of the information contained in this document. Use of the information in this document remains the sole responsibility of the reader. Although, the information provided on this Q&A document has been prepared with the utmost care, possible errors or omissions cannot be excluded. The European Chemicals Agency and the authorities from the five countries does not accept any liability with regards to any such errors or omissions. P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | echa.europa.eu Question Answer General process We expect to notify the proposal in ECHA's registry of intention (RoI) in the first half of 2021 and to submit the completed assessment and proposal to ECHA for opinion making in the first half of 2022. -

FKM Xplor™ V9T82
TRELLEBORG SEALING SOLUTIONS FKM XploRTM V9T82 EXPLOSIVE DECOMPRESSION RESISTANT MATERIALS OUTSTANDING EXTREME LOW TEMPERATURE PERFORMANCE Explosive decompression is a major concern to the oil and gas industry. It occurs when applied system pressure is released, causing absorbed gas to expand, potentially damaging elastomer seals. Trelleborg Sealing Solutions has focused on this issue and Features and benefits presents the XploR™ range, an entire family of advanced elastomers especially developed for oil and gas applications. • Unrivalled ED restistance within its material type The portfolio includes compounds in HNBR, FKM, Aflas® and • Operating temperatures from -48°C to +200°C/-54°F to Isolast® Perfluoroelastomer, each of which demonstrates +392°F with short excursions to 210°C/+410°F best-in-class Explosive Decompression (ED) resistance for • Outstanding performance at extremely low temperatures its material type. • Exceptional mechanical performance • Low long-term compression set XploR™ V9T82 combines excellent chemical and thermal • Very good chemical compatibility properties, with outstanding low temperature capability. • Extended life in aggressive media, including the hydrocarbon It exhibits superior high pressure sealing performance in and aqueous media common within oil & gas applications ED situations, which is supported by independent institute • High modulus and high strength approval to standard test protocols. Applications When the composition of the well or conditions of the application are known, FKM XploR™ V9T82 may prove the • Separation equipment optimum and most cost-effective material for your application, • Connector systems especially when operating temperatures are extremely low. • Valves • Wellhead control equipment For further information on selecting the right compound and • Tubing hangers advice on seal specification for your individual application, • Swivel stacks on Floating Production Storage and consult your local Trelleborg Sealing Solutions marketing Offloading (FPSO) vessels company. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Acrylamide Polymerization — a Practical Approach
electrophoresis tech note 1156 Acrylamide Polymerization — A Practical Approach Paul Menter, Bio-Rad Laboratories, 2000 Alfred Nobel Drive, Polyacrylamide Gel Polymerization Hercules, CA 94547 USA AcrylamideBis Polyacrylamide Introduction The unparalleled resolution and flexibility possible with CH2 CH + CH2 CH CH2 CH CH2 CH CH2 CH polyacrylamide gel electrophoresis (PAGE) has led to its CO CO CO CO CO widespread use for the separation of proteins and nucleic NH2 NH NH2 NH2 NH acids. Gel porosity can be varied over a wide range to meet CH2 CH2 specific separation requirements. Electrophoresis gels and NH NH NH NH buffers can be chosen to provide separation on the basis of CO 2 2 CO CO C O charge, size, or a combination of charge and size. CH2 CH CH2 CH CH2 CH CH2 CH The key to mastering this powerful technique lies in the polymerization process itself. By understanding the important Purity of Gel-Forming Reagents parameters, and following a few simple guidelines, the novice Acrylamide can become proficient and the experienced user can optimize Gel-forming reagents include the monomers, acrylamide and bis, separations even further. as well as the initiators, usually ammonium persulfate and TEMED or, occasionally, riboflavin and TEMED. On a molar This bulletin takes a practical approach to the preparation of basis, acrylamide is by far the most abundant component in the polyacrylamide gels. Its purpose is to provide the information monomer solution. As a result, acrylamide may be the primary required to achieve reproducible, controllable polymerization. source of interfering contaminants (Dirksen and Chrambach For those users interested only in the “bare essentials,” the 1972). -

(Ph.D.) Environmental Engineering
UNIVERSITY OF CINCINNATI Date: June 15, 2005 I, Georgios Anipsitakis , hereby submit this work as part of the requirements for the degree of: Doctorate of Philosophy (Ph.D.) in: Environmental Engineering It is entitled: Cobalt/Peroxymonosulfate and Related Oxidizing Reagents for Water Treatment This work and its defense approved by: Chair: Dr. Dionysios Dionysiou Dr. Paul Bishop Dr. George Sorial Dr. Souhail Al-Abed COBALT/PEROXYMONOSULFATE AND RELATED OXIDIZING REAGENTS FOR WATER TREATMENT A dissertation submitted to the Division of Research and Advanced Studies of the University of Cincinnati in partial fulfillment of the requirements for the degree of DOCTORATE OF PHILOSOPHY (Ph.D.) in the Department of Civil and Environmental Engineering of the College of Engineering 2005 by Georgios P. Anipsitakis Diploma (B.S./M.S.), Chemical Engineering, Nat. Technical Univ. Athens, 2000 Committee Chair: Dr. Dionysios D. Dionysiou ii Abstract This dissertation explores the fundamentals of a novel advanced oxidation technology, the II cobalt/peroxymonosulfate (Co /KHSO5) reagent, for the treatment of persistent and hazardous II substances in water. Co /KHSO5 is based on the chemistry of the Fenton Reagent and proceeds via the generation of sulfate radicals, which similarly to hydroxyl radicals, readily attack and degrade organic and microbial contamination in water. Very few studies have exploited the reactivity of sulfate radicals for environmental applications. Compared to the extensively investigated hydroxyl radicals, sulfate radicals are not fully understood. Following this approach, the coupling of nine transition metals with hydrogen peroxide (H2O2), potassium peroxymonosulfate (KHSO5) and persulfate (K2S2O8) was also explored. The objective was again the generation of inorganic radicals and the efficient degradation of organic contaminants in water. -

Comprehensive Testing to Measure the Jtespoi^Se^Yf Jhuorocarbdn Rubber (FKM) Whanfomtank1 Waste Simulant
2t 2000 SANDIA REPORT SAND2000-032SA 5 i Unlimited Release Rrinted February 2000 Comprehensive Testing to Measure the jtespoi^se^yf JHuorocarbdn Rubber (FKM) WHanfoMTank1 Waste Simulant Paul J. Nigrey and DenniS'L. Bolton Prepared by Sandia National Laboratories Albuquerque, New Mexico 87185 and Livermore, California 94550 Sandia'is a multiprogram laboratory operated by Sandia Corporation, a Lock'heed Martin Company, for the United States Department of Energy under Contract DE-AC04-94AL85000. Approved for public release; further dissemination unlimited. Sandia National Laboratories Issued by Sandia National Laboratories, operated for the United States Department of Energy by Sandia Corporation. NOTICE: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government, nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represent that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government, any agency thereof, or any of their contractors or subcontractors. The views and opinions expressed herein do not necessarily state or reflect those of the United States Government, any agency thereof, or any of their contractors. Printed in the United States of America. This report has been reproduced directly from the best available copy. -

Xyfluor Chemical Compatibility Guide
456456 Xyfluor® Chemical Compatibility Xyfluor® is a proprietary, highly fluorinated elastomer. The oxygen in the polymer backbone provides outstanding low-temperature capabilities - far better than FKM or FFKM elastomers. The polymer provides improved resistance to many harsh chemicals that can attack the hydrogen in FKM elastomers. The chemical resistance of Xyfluor® approaches but is not equivalent to FFKM elastomers. A = Swell < 10% after exposure. Suitable. B = Swell > 10% & < 20% after exposure. Generally suitable. C = Swell >20% & < 40% after exposure. May be suitable in some situations. D = Swell > 40% after exposure. Not suitable. N = Insufficient data. Test: Full immersion, Room Temperature, 3 days The information contained herein is believed to be reliable, but no representation, guarantees or warranties of any kind are made to its accuracy or suitability for any purpose. Full-scale testing and end-product performance are the responsibility of the user. CHEMICAL RATING CHEMICAL RATING acetaldehyde A amomnium phosphate A acetic acid, ammonium stearate A glacial A ammonium sulfate A hot A ammonium thiocyanate A 5% A amyl acetate A/B acetic anhydride A amyl alcohol A acetone A amyl nitrate A acetone cyanohydrin A aniline A acetyl chloride A aniline hydrochloride A acetylene gas A anti-freeze, alcohol or glycol based A acrylonitrile A aqua regia N adipic acid A argon gas A alcohol, denatured A arsenic acid A alkyl benzene A ash slurry A alkyl-arylsulphonic acid A asphalt A alumina trihydrate N barium chloride A aluminum acetate -

Microchip Design for Chemiluminesence Detection
Double spiral detection channel for on-chip chemiluminescence detection Author Lok, Khoi Seng, Kwok, Yien Chian, Nam-Trung, Nguyen Published 2012 Journal Title Sensors and Actuators B: Chemical DOI https://doi.org/10.1016/j.snb.2012.04.047 Copyright Statement © 2012 Elsevier B.V.. This is the author-manuscript version of this paper. Reproduced in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/50085 Griffith Research Online https://research-repository.griffith.edu.au Lab on a Chip for Chemiluminescence Detection Khoi Seng Lok1, Yien Chian Kwok1* and Nam Trung Nguyen2 1National Institute of Education, Nanyang Technological University, Nanyang Walk, Singapore 637616, Singapore. E-mail: [email protected] 2School of Mechanical and Production Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. E-mail: [email protected] Abstracts In this paper reports a three layered device that consists of a double spiral channel design for chemiluminsence (CL) detection and a passive micromixer to facilitate mixing of reagents. Compared to the design with a single spiral channel, the design with two overlapping spiral channels doubled the CL intensity emitted from the reaction. Furthermore, the addition of the passive micromixer improved the signal by 1.5 times. Luminol-based chemiluminescence reaction was used for the characterization of the device. This device was successfully used for the determination of L-cysteine and uric acid. Keywords: chemiluminescence, lab-on-chip, micro total analysis system, luminol, cobalt, L-cysteine, uric acid 1 Introduction Chemiluminescence (CL) detection methods do not require an excitation light source. -

Hydrogen Peroxide Material Compatibility Chart from ISM and IS
ver 09-Jul-2020 Hydrogen Peroxide Material Compatibility Chart All wetted surfaces should be made of materials that are compatible with hydrogen peroxide. The wetted area or surface of a part, component, vessel or piping is a surface which is in permanent contact with or is permanently exposed to the process fluid (liquid or gas). Less than 8% concentration H2O2 is considered a non-hazardous substance. Typically encountered versions are baking soda-peroxide toothpaste (0.5%), contact lens sterilizer (2%), over-the-counter drug store Hydrogen Peroxide (3%), liquid detergent non-chlorine bleach (5%) and hair bleach (7.5%). At 8% to 28% H2O2 is rated as a Class 1 Oxidizer. At these concentrations H2O2 is usually encountered as a swimming pool chemical used for pool shock treatments. In the range of 28.1% to 52% concentrations, H2O2 is rated as a Class 2 Oxidizer, a Corrosive and a Class 1 Unstable (reactive) substance. At these concentrations, H2O2 is considered industrial strength grade. Concentrations from 52.1% to 91% are rated as Class 3 Oxidizers, Corrosive and Class 3 Unstable (reactive) substances. H2O2 at these concentrations are used for specialty chemical processes. At concentrations above 70%, H2O2 is usually designated as high-test peroxide (HTP). Concentrations of H2O2 greater than 91% are currently used as rocket propellant. At these concentrations, H2O2 is rated as a Class 4 Oxidizer, Corrosive and a Class 3 Unstable (reactive) substance. Compatibility Compatibility Compatibility Compatibility Material 10% H2O2 30% H2O2 50% -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Pvc Piping Systems for Commercial and Industrial Applications
PVC PIPING SYSTEMS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONS Plastic Pipe and Fittings Association © 2012 Plastic Pipe and Fittings Association (PPFA) Acknowledgments We would like to thank the following contributors to the Design Guide: The PVC and Thermoplastic Industrial Piping Systems (TIPS) Product Line Committees and member companies of the Plastic Pipe and Fittings Association (PPFA). In particular the following PPFA companies and individuals ably assisted in reviewing the text and tables and provided valuable comments which added greatly in producing a better and more accurate source document: Chuck Bush – Oatey Company Mike Cudahy – PPFA Staff Patrick Fedor – IPEX Bill Morris – Charlotte Pipe & Foundry Jack Roach – Mueller Industries Bill Weaver – Harvel Plastics Larry Workman – LASCO Fittings All text, tables and photos were prepared and or edited by David A. Chasis of Chasis Consulting, Inc. Using the Design Guide The Design Guide was created to assist engineers, installers, end-users, engineering students and building code officials in learning more of the dos and don’ts of PVC piping systems. The Design Guide is comprised of ten sections including: Introduction Features and Benefits Engineering Design Joining Methods Installation Testing and Repair Applications Building Codes, Standards, and Sample Specifications PVC Piping and the Environment Other Plastic Piping Systems In addition, in the back of the guide is the most complete appendix and glossary of PVC piping systems ever assembled. Other PPFA Educational Materials The PPFA offers a wide range of other educational materials developed to assist the engineering and construction industry to become more proficient in the use of the preferred piping system...plastics! On-site seminars, Webinars, CD-based seminars, workbooks, online tutorials and product and technical literature are available. -
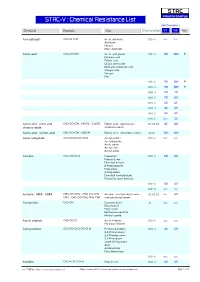
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r.