(12) Patent Application Publication (10) Pub. No.: US 2011/005250.6 A1 Abel Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Rubber and Composite Hose Chemical Resistant Chart
Rubber and Composite Hose Chemical Resistant Chart Cedar Rapids, IA Corporate Headquarters Phone: 319.365.0471 Toll Free: 800.553.5455 Fax: 319.365.2522 Website: www.apache-inc.com 99004032 rev032713 Chemical Resistance Information This Apache document provides essential information that will facilitate the safe use of rubber and composite type chemical hoses. Chemical hose users are cautioned that this Chemical Resistant has be developed from generallty accepted industry standards. The ratings listed beneath each Elastomer are the base ratings for the chemical listed. This rating is based on the application temperature not exceeding 70ºF (21.1ºC) unless otherwise specified. The degree an Elastomer will resist the effects of a of a specific chemical depends on several variables.It is recommended that a hose with the highest resistant tube to the chemical transferred be used in the application for safety. 1. Concentration of the chemical is very significant (some chemicals may react with an Elastomer differently based on the level of concentration). 2. Temperature - as the temperature increases the deteriorative effect of a chemical may greatly increase on an Elastomer. 3. Time - the longer the duration the chemical is in contact with the Elastomer, the greater the deteriorative effect. 4. Stability of the Chemical - Chemical solutions (combining of different chemicals) may increase the deteriorative effect. 5. Elastomer Grade - There are different grades of specific Elastomer used in hose. The grade of Elastomer used may effect the resistance level of the hose to a specific chemical. It is recommended that only hose listed for chemical service be use. 6. Safety a. -
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
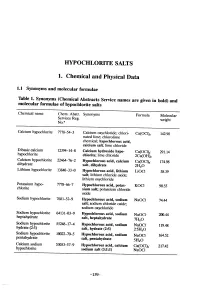
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Inventory of Biocides Used in Denmark
Environmental Project No. 585 2001 Miljøprojekt Inventory of Biocides used in Denmark Carsten Lassen, Susanne Skårup, Sonja Hagen Mikkelsen og Jesper Kjølholt COWI Pia Juul Nielsen Danish Toxicology Centre Lise Samsøe-Petersen DHI - Water & Environment The Danish Environmental Protection Agency will, when opportunity offers, publish reports and contributions relating to environmental research and development projects financed via the Danish EPA. Please note that publication does not signify that the contents of the reports necessarily reflect the views of the Danish EPA. The reports are, however, published because the Danish EPA finds that the studies represent a valuable contribution to the debate on environmental policy in Denmark. Table of Contents SUMMARY 5 DANSK SAMMENFATNING 9 1 INTRODUCTION 13 2 METHODOLOGY 15 3 MAIN GROUP 1: DISINFECTANTS AND GENERAL BIOCIDAL PRODUCTS 18 3.1 PRODUCT-TYPE 1: HUMAN HYGIENE BIOCIDAL PRODUCTS 18 3.1.1 Skin disinfectants 19 3.2 PRODUCT-TYPE 2: PRIVATE AREA AND PUBLIC HEALTH AREA DISINFECTANTS AND OTHER BIOCIDAL PRODUCTS 23 3.2.1 Disinfectants for private areas 23 3.2.2 Disinfectants for professional cleaning and industrial use 24 3.2.3 Disinfectants for medical equipment 27 3.2.4 Disinfectants for laundries 29 3.2.5 Disinfectants for air-conditioning system 30 3.2.6 Disinfectants for chemical toilets 30 3.2.7 Disinfectants for swimming pools 31 3.2.8 Disinfectants for wastewater and hospital waste 32 3.3 PRODUCT-TYPE 3: VETERINARY HYGIENE BIOCIDAL PRODUCTS 33 3.3.1 Disinfectants applied directly to -

Korea Industrial Tariff Schedule
DRAFT Subject to Legal Review for Accuracy, Clarity, and Consistency Annex 2-B Industrial Schedule for the Republic of Korea Staging HSK 10 Description Base Rate Category 0301101000 Gold carp 10 C 0301102000 Tropical fish 10 C 0301109000 Other 10 A 0301911000 Salmo trutta, Oncorhynchus mykiss,Oncorhynchus clarki, Oncorhynchus 10 C aguabonita,Oncorhynchus gilae 0301912000 Oncorhynchus apache and Oncorhynchus chrysogaster 10 C 0301921000 Glass eel 10 A 0301929000 Other 30% or I ₩1,908/kg 0301930000 Carp 10 C 0301992000 Yellow tail 10 A 0301994000 Sea-bream 45% or G ₩3,292/kg 0301995000 Conger eel 10 G 0301996000 Sharp toothed eel 10 G 0301997000 Salad eel 10 C 0301998000 Flat fish 10 C 0301999010 True bass 10 G 0301999020 Puffers 10 G 0301999030 Tilapia 10 C 0301999040 Rock fish(including pacific ocean perch) 10 C 0301999050 Sea bass 40 C 0301999060 Mullets 10 C 0301999070 Loaches 10 C 0301999080 Cat fishes 10 A 0301999091 Rock Trout(Hexagrammos spp., Agrammus spp.) 10 C 0301999092 Crusian carp 10 C 0301999093 Salmon 10 A 0301999094 Grass carp 10 A 0301999095 Croakers 36 G 0301999099 Other 10 G 0302111000 Salmo trutta, Oncorhynchus mykiss, Oncorhynchus clarki,Oncorhynchus 20 G aquabonita, Oncorhynchus gilae 0302112000 Oncorhynchus apache and Oncorhynchus chrysogaster 20 A 0302120000 Pacific salmon(Oncorhynchus nerka, Oncorhynchus gorbuscha, Oncorhynchus 20 A keta, Oncorhynchus tschawytscha, Oncorhynchus kisutch, Oncorhynchus masou and Oncorhynchus rhodurus), Atlantic salmon(Salmo salar)and Danube salmon(Hucho hucho) 0302190000 Other 20 -

United States Patent Office Patented July 18, 1972 2 Linear Or Branched in Its Carbon Backbone, and One to 3,677,952 Two Sulfonate Groups Per Aromatic Nucleus
4.d4 Z v O. f U - bs 2 XR 3 g6 7.7 g 952 3,677,952 United States Patent Office Patented July 18, 1972 2 linear or branched in its carbon backbone, and one to 3,677,952 two sulfonate groups per aromatic nucleus. Representa METHOD OF BLEACHING SULFONATE tive of this class are dodecylbenzenesulfonic acid, octa SURFACTANTS decylnaphthalenesulfonic acid, decyltoluenesulfonic acid, Lowell B. Lindy, Midland, Mich., assignor to The Dow decyldiphenyl ether disulfonic acid, dodecyldiphenyl ether Chemical Company, Midland, Mich. 5 disulfonic acid, dodecylchlorodiphenyl ether disulfonic No Drawing. Filed Oct. 2, 1970, Ser. No. 77,745 acid, and the sodium and potassium salts of these com Int, C. C11d 7/54 pounds. U.S. C. 252-94 7 Claims The process is particularly adapted to efficient de 10 colorizing of alkyldiphenyl ether disulfonates having an ABSTRACT OF THE DISCLOSURE average of about 1-1.3 alkyl groups of 8-18 carbon atoms and an average of about 1.6-2 sulfonate groups Alkylaryl sulfonate surfactants are bleached by react per diphenyl ether nucleus. ing their aqueous solutions with an inorganic hypohalite By the terms inorganic hypohalite and inorganic per and an inorganic permanganate, sequentially or in com manganate are meant hypochlorous acid, hypobromous bination. The process is particularly adaptable to de 5 acid, permanganic acid and the anhydrides and water colorizing higher alkyl diphenyl ether disulfonates using soluble inorganic salts of these, particularly the sodium sodium hypochlorite and potassium permanganate as the and potassium salts. In practice, sodium hypochlorite and bleaching agents. potassium permanganate are used since these are cheap 20 est and most readily available. -

November 21, 2017 OPP Docket Environmental Protection Agency Docket Center (EPA/DC) Mail Code (28221T) 1200 Pennsylvania Ave. NW
Government Affairs Office 1300 Eye Street NW Suite 701W Washington, DC 20005-3314 T 202.628.8303 F 202.628.2846 November 21, 2017 OPP Docket Environmental Protection Agency Docket Center (EPA/DC) Mail Code (28221T) 1200 Pennsylvania Ave. NW Washington, DC 20460‐0001 RE: Registration Review Proposed Interim Decisions for Several Pesticides; Notice of Availability; Sodium, Calcium, and Potassium Hypochlorites, Case Numbers 0029, 5076. Docket ID# EPA–HQ–OPP–2012–0004 and EPA–HQ–OPP–2014–0157 Dear Ms. Bailey, The American Water Works Association would like to thank the U.S. Environmental Protection Agency for the opportunity to comment on the Agency’s proposed Interim Decision regarding Case Numbers 0029 and 5076. AWWA reviewed the proposed interim decision and offers the following recommendations: It is appropriate for EPA to take this interim decision using the available data. The Federal Insecticide Fungicide and Rodenticide Act (FIFRA) label requirements for hypochlorites must not interfere with application of disinfectants as required and currently implemented under the Safe Drinking Water Act. The proposed product label precautionary statements are sound and provide a mechanism to reduce the formation of chlorate and perchlorate contamination in hypochlorite solutions used to disinfect drinking water. Specific comments on the proposed interim decision are described below: Reconciling SDWA and FIFRA Chlorine Residual Requirements The interim decision points out FIFRA label language for hypochlorite use that is at odds with drinking water disinfection requirements under the Safe Drinking Water Act. “In 1998, pursuant to the Safe Drinking Water Act, EPA’s Office of Water set a Maximum Residual Disinfectant Level Goal (MRDLG) of 4 mg/L and a Maximum Residual Disinfectant Level (MRDL) of 4 mg/L for chlorine based on a running annual mean for all measurements taken in the distribution system. -

Maximum Usage Temperatures for KYNAR (PVDF)
Maximum Usage Temperatures for KYNAR (PVDF) *NR Indicated KYNAR is not recommended for use *Temperatures listed do not take into account vacuum, mixtures, and close tolerances Chemical Substance Concentration °F °C Acetaldehyde NR Acetamide 75 25 Acetic Acid 120 50 Acetic Acid 10% in water 225 110 Acetic Acid 50% in water 200 95 Acetic Acid 80% in water 150 65 Acetic Anhydride NR Acetone NR Acetone 10% in water 125 50 Acetonitrile 125 50 Acetophenone NR Acetyl Bromide 125 50 Acetyl Chloride 125 50 Acetylacetone NR Acetylene 250 120 Acrylonitrile 75 25 Adipic Acid 150 65 Air 285 140 Alcoholic Spirits 40% Ethyl Alcohol 200 95 Allyl Alcohol 125 50 Allyl Chloride 212 100 Aluminum Acetate Aqueous Solution or Solid 285 140 Aluminum Bromide 285 140 Aluminum Chloride up to 40% in water 285 140 Aluminum Fluoride Aqueous Solution or Solid 275 135 Aluminum Hydroxide 275 135 Aluminum Nitrate Aqueous Solution or Solid 275 135 Aluminum Oxychloride 275 135 Aluminum Sulfate Aqueous Solution or Solid 275 135 Ammonia, Gas NR Ammonia, Liquid NR Ammonium Acetate Aqueous Solution or Solid 175 80 Ammonium Alum Aqueous Solution or Solid 275 135 Ammonium Bifluoride Aqueous Solution or Solid 150 65 Ammonium Bromide Aqueous Solution or Solid 250 120 Ammonium Carbonate Aqueous Solution or Solid 275 135 Ammonium Chloride Aqueous Solution or Solid 275 135 Ammonium Dichromate Aqueous Solution or Solid 250 120 Ammonium Fluoride Aqueous Solution or Solid 275 135 Ammonium Hydroxide up to "Concentrated" 225 110 Ammonium Metaphosphate Aqueous Solution or Solid 275 135 Ammonium -

NEOPRENE Compatibility Guide
CHEMICAL COMPATIBILITY CHART NEOPRENE Our products can be exposed to a huge variety of chemicals. The data table below is an application guide, and indicates the resistance of the specific thermoplastics we use in the construction of our products, to common chemicals. The data given should be used cautiously, and as a guide only. Various factors such as concentration, additives, exposure time, temperature and internal mechanical stress levels will all impact on the working life of our plastic parts. Use the table conservatively and if any doubt exists, do not proceed with the application. In the table below there are four ratings: • A-Excellent indicates that at ambient temperature and pressure, the material should not be affected. • B-Good indicates that the material is slightly affected but not to the point of being unsuitable. • C-Fair indicates a degree of reaction that is generally considered unsuitable and should not be used. • D-Severe Effect indicates that the material should not be used under any circumstances All ratings are taken from data measured at ambient temperature and pressure. Page 1 of 13 Kelco 15/12/2008 CHEMICAL COMPATIBILITY Acetaldehyde C-Fair Acetamide B-Good Acetate Solvent D-Severe Effect Acetic Acid C-Fair Acetic Acid 20% A-Excellent Acetic Acid 80% C-Fair Acetic Acid, Glacial D-Severe Effect Acetic Anhydride A-Excellent Acetone C-Fair Acetyl Chloride (dry) D-Severe Effect Acetylene B-Good Acrylonitrile C-Fair Adipic Acid C-Fair Alcohols:Amyl A-Excellent Alcohols:Benzyl C-Fair Alcohols:Butyl A-Excellent Alcohols:Diacetone -

(12) United States Patent (10) Patent No.: US 9.233,262 B2 Pond Et Al
USOO9233262B2 (12) United States Patent (10) Patent No.: US 9.233,262 B2 Pond et al. (45) Date of Patent: Jan. 12, 2016 (54) DENTAL IRRIGANT 5,719,113 A * 2/1998 Fendler et al. ................ 510,382 5,739,168 A * 4/1998 Hioki et al. ... ... 514,643 6, 190,542 B1* 2/2001 Comolli et al. ... ... 208/423 (75) Inventors: ARAG, Sw (US) 6,190,642 B1* 2/2001 Dougherty et al. ............. 424/49 s s 6.255.267 B1* 7/2001 Nayar et al. ....... ... 510,191 6,323,171 B1 * 1 1/2001 Fonsny et al. ................. 510,384 (73) Assignee: Inter-Med, Inc., Racine, WI (US) 7,063,793 B2 6/2006 Albiston et al. 7,070,737 B2 * 7/2006 Bains et al. ..................... 422/37 (*) Notice: Subject to any disclaimer, the term of this 3886. A. : 239: IIKennyth etstal al. .. 39. past ised fos lusted under 35 2004f0071748 A1* 4/2004 Asmus et al. ...... ... 424/401 .S.C. 154(b) by yS. 2006/0241011 A1* 10, 2006 Renfrow ....................... 510,499 (21) Appl. No.: 12/217,601 FOREIGN PATENT DOCUMENTS (22) Filed: Jul. 7, 2008 WO WOO3,061506 T 2003 (Under 37 CFR 1.47) OTHER PUBLICATIONS (65) Prior Publication Data Surface tension conversion, 2011. US 2009/OO88476A1 Apr. 2, 2009 Cameron, Australian Dental Journal, 31, 1996. Jeansonne, Journal of Endodontics, 20, 1996. Related U.S. Application Data Abou-Rass, Oral Surg., 53, 1982.* Shen et al., “Antimicrobial Efficacy of Chlorhexidine Against Bac (63) y of PE No. 11/998,972, filed on teria in Biofilms at Different Stages of Development'; JOE vol. -

PVDF Chemical Resistance Guide
PVDF Chemical Resistance Guide SECOND EDITION PVDF CHEMICAL RESISTANCE GUIDE Thermoplastics: Kynar® Polyvinylidene Fluoride (PVDF) for Waste Drainage Systems Chemical Resistance Guide Kynar® Polyvinylidene Fluoride (PVDF) for Waste Drainage Systems 2nd Edition © 2021 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 About IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Thermoplastics and elastomers have outstanding resistance to a wide range of chemical reagents. The chemical resistance of plastic piping is basically a function of the thermoplastic material and the compounding components.