FPM Chemical Resistance Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

RFC: IRIS Barium and Compounds Substance File
October 29, 2002 Information Quality Guidelines Staff Mail Code 28221T U.S. EPA 1200 Pennsylvania Ave., N.W. Washington, DC, 20460 Subject: Request for Correction of the IRIS Barium and Compounds substance file - Information disseminated by EPA that does not comply with EPA or OMB Information Quality Guidelines Dear Madam or Sir; Chemical Products Corporation (CPC), a Georgia corporation which produces Barium and Strontium chemicals at its Cartersville, Georgia facility, hereby submits this Request for Correction (RFC) concerning EPA’s Integrated Risk Information System Barium and Compounds Substance File (IRIS Ba File). The influential information contained in this file fails to comply with the OMB “Guidelines for Ensuring and Maximizing the Quality, Objectivity, Utility, and Integrity of Information Disseminated by Federal Agencies”. The information disseminated in EPA’s IRIS Barium and Compounds file directly contradicts the information published by EPA in the January 3, 1997 Federal Register and, therefore, cannot represent an EPA consensus position. The IRIS Ba File was revised in 1998 and 1999, yet it contains no mention of the toxicological evaluation conducted by EPA’s Office of Pollution, Pesticides, and Toxic Substances reported in 62 FR 366-372 (No. 2, January 3, 1997). There is no explanation of how a radically different interpretation of the same data could be justified. The NOAEL employed to calculate the Oral Reference Dose in the IRIS Ba File is 0.21 mg/kg/day; there is no LOAEL associated with this NOAEL. The NOAEL reported in 62 FR 366-372 is 70 mg/kg/day in rats and 165 mg/kg/day in mice; these values are taken from a National Toxicology Program technical report and are associated with a LOAEL of 180 mg/kg/day. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

WO 2015/025175 Al 26 February 2015 (26.02.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/025175 Al 26 February 2015 (26.02.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C09K 5/06 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/GB2014/052580 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 22 August 2014 (22.08.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13 15098.2 23 August 2013 (23.08.2013) GB (84) Designated States (unless otherwise indicated, for every (71) Applicant: SUNAMP LIMITED [GB/GB]; Unit 1, Satel kind of regional protection available): ARIPO (BW, GH, lite Place, Macmerry, Edinburgh EH33 1RY (GB). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (72) Inventors: BISSELL, Andrew John; C/o SunAmp, Unit TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, 1, Satellite Place, Macmerry, Edinburgh EH33 1RY (GB). -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -

Pvc Piping Systems for Commercial and Industrial Applications
PVC PIPING SYSTEMS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONS Plastic Pipe and Fittings Association © 2012 Plastic Pipe and Fittings Association (PPFA) Acknowledgments We would like to thank the following contributors to the Design Guide: The PVC and Thermoplastic Industrial Piping Systems (TIPS) Product Line Committees and member companies of the Plastic Pipe and Fittings Association (PPFA). In particular the following PPFA companies and individuals ably assisted in reviewing the text and tables and provided valuable comments which added greatly in producing a better and more accurate source document: Chuck Bush – Oatey Company Mike Cudahy – PPFA Staff Patrick Fedor – IPEX Bill Morris – Charlotte Pipe & Foundry Jack Roach – Mueller Industries Bill Weaver – Harvel Plastics Larry Workman – LASCO Fittings All text, tables and photos were prepared and or edited by David A. Chasis of Chasis Consulting, Inc. Using the Design Guide The Design Guide was created to assist engineers, installers, end-users, engineering students and building code officials in learning more of the dos and don’ts of PVC piping systems. The Design Guide is comprised of ten sections including: Introduction Features and Benefits Engineering Design Joining Methods Installation Testing and Repair Applications Building Codes, Standards, and Sample Specifications PVC Piping and the Environment Other Plastic Piping Systems In addition, in the back of the guide is the most complete appendix and glossary of PVC piping systems ever assembled. Other PPFA Educational Materials The PPFA offers a wide range of other educational materials developed to assist the engineering and construction industry to become more proficient in the use of the preferred piping system...plastics! On-site seminars, Webinars, CD-based seminars, workbooks, online tutorials and product and technical literature are available. -
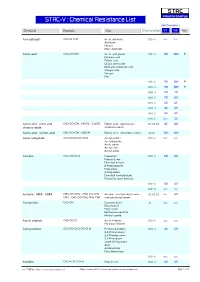
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Whole Foods Market Unacceptable Ingredients for Food (As of March 15, 2019)
Whole Foods Market Unacceptable Ingredients for Food (as of March 15, 2019) 2,4,5-trihydroxybutyrophenone (THBP) benzoyl peroxide acesulfame-K benzyl alcohol acetoin (synthetic) beta-cyclodextrin acetone peroxides BHA (butylated hydroxyanisole) acetylated esters of mono- and diglycerides BHT (butylated hydroxytoluene) activated charcoal bleached flour advantame bromated flour aluminum ammonium sulfate brominated vegetable oil aluminum potassium sulfate burnt alum aluminum starch octenylsuccinate butylparaben aluminum sulfate caffeine (extended release) ammonium alum calcium benzoate ammonium chloride calcium bromate ammonium saccharin calcium disodium EDTA ammonium sulfate calcium peroxide apricot kernel/extract calcium propionate artificial sweeteners calcium saccharin aspartame calcium sorbate azo dyes calcium stearoyl-2-lactylate azodicarbonamide canthaxanthin bacillus subtilis DE111 caprocaprylobehenin bacteriophage preparation carmine bentonite CBD/cannabidiol benzoates certified colors benzoic acid charcoal powder benzophenone Citrus Red No. 2 Page 1 of 4 cochineal foie gras DATEM gardenia blue diacetyl (synthetic) GMP dimethyl Silicone gold/gold leaf dimethylpolysiloxane heptylparaben dioctyl sodium sulfosuccinate (DSS) hexa-, hepta- and octa-esters of sucrose disodium 5'-ribonucleotides high-fructose corn syrup/HFCS disodium calcium EDTA hjijiki disodium dihydrogen EDTA hydrogenated oils disodium EDTA inosine monophosphate disodium guanylate insect Flour disodium inosinate iron oxide dodecyl gallate kava/kava kava EDTA lactic acid esters of monoglycerides erythrosine lactylated esters of mono- and diglycerides ethoxyquin ma huang ethyl acrylate (synthetic) methyl silicon ethyl vanillin (synthetic) methylparaben ethylene glycol microparticularized whey protein derived fat substitute ethylene oxide monoammonium glutamate eugenyl methyl ether (synthetic) monopotassium glutamate FD&C Blue No. 1 monosodium glutamate FD&C Blue No. 2 myrcene (synthetic) FD&C Colors natamycin (okay in cheese-rind wax) FD&C Green No. -

Thermoplastic Composite Chemical Compatibility Guide
123 WR ®300/525/600, AR ®HT & ARLON ® 1000 Chemical Resistance Data WR®300/525, AR®HT Concentrate & ARLON® 1000 Chemical Weight % <275°F (135°C) >275°F (135°C) WR®600 Comments Acetaldehyde, aq. 40 111 Acetamide, aq. 50 111 Acetic Acid, aq. 10 111 Acetic Acid, glacial 100 121 Acetic Anhydride 11Material 1 may react with absorbed moisture Acetone 1 2 – 3 1 Mechanical properties loss <30% for material 2 at T>120 ºF (50 ºC) Acetonitrile 11 Acetophenone 2 3 – 4 1 Material 1 may be slightly soluble at temperatures >390ºF (200ºC) Acetylene 11 Acetyl Chloride, dry 11Material 1 may react with absorbed moisture Acids-Aliphatic, pure 1 1 – 2 1 Acids-Sulfonic, pure 2 – 4 3 – 4 1 Acids-Non oxidizing, aq. <40 1 1 – 2 1 Acrylic Acid 111 Acrylonitrile 11 Adipic Acid 11 Air 1 2 – 3 1 Oxidation for material 1 increases at higher temperatures Alcohols, Aliphatic 111 Allyl Chloride 2 2 – 3 1 Allyl Alcohol 111 Aluminum Chloride, aq. 10 111 Aluminum Chloride, anydrous 441 Aluminum Nitrate, aq. Sat. 11 Aluminum Sulfate, aq. 10 111 Ammonia, aq. Conc. 111 Ammonia, Liquid 11 Ammonia Gas 111 Ammonium Carbonate, aq. 10 111 Ammonium Chloride, aq. 10 111 Ammonium Chloride, aq. 37 111 Ammonium Fluoride, aq. Sat. 11 Ammonium Hydroxide 11 Notes: Material 1= WR300, WR525, ARHT & Arlon 1000 Material 2= WR600 www.gtweed.com WR®300/525, AR®HT Concentrate & ARLON® 1000 Chemical Weight % <275°F (135°C) >275°F (135°C) WR®600 Comments Ammonium Nitrate, aq. Sat. 11 Ammonium Phosphate, aq. Sat. -
![[Technical Information] Aluminium Sulphate – 200 Mesh Polysol](https://docslib.b-cdn.net/cover/1585/technical-information-aluminium-sulphate-200-mesh-polysol-901585.webp)
[Technical Information] Aluminium Sulphate – 200 Mesh Polysol
[Technical Information] Aluminium sulphate – 200 Mesh Polysol Industries Plot no.-C1B-106/1 to 106/4, G.I.D.C, Sarigam(Gujarat)-INDIA Tel-091-0260-2431587, E-mail: [email protected] Aluminium sulfate is mainly use as a flocculating agent in the purification of drinking water and waste water treatment plants, and also in paper manufacturing. It is widely use in the concrete technology as accelerating agent. Nature: Aluminium sulfate, is a chemical compound with the formula Al2(SO4)3 14-18H2O CAS Number 7784-31-8 Specification: Form : Powder Appearance : White fine free flowing Powder pH value (1:10) : Min. 2.0 Solubility : Completely soluble in hot water. Al as Al2O3 : Min. 15 % Insoluble matter : Max. 0.8 % (In water) Iron content as Fe : Below 50 ppm Particle size (Retention on) 100 Mesh sieve : Max 2.0 % 200 Mesh sieve : Max 35.0 % 300 Mesh sieve : Max 95.0 % Properties: Aluminium sulfate, alternatively spelt either aluminum or sulphate, is a chemical compound with the formula Al2(SO4)3. Aluminium sulfate is sometimes referred to as a type of alum. Application: Sizing paper, lakes, mordent for drying, foaming agent in fire foams for leather and clarifying agent for fat and oil. Aluminium sulfate is used in water purification and as a mordant in dyeing and printing textiles. In water purification, it causes impurities to coagulate which are removed as the particulate settles to the bottom of the container or more easily filtered. This process is called coagulation or flocculation.When dissolved in a large amount of neutral or slightly alkaline water, aluminium sulfate produces a gelatinous precipitate of aluminium hydroxide, Al(OH)3. -

Toxicological Profile for Barium and Barium Compounds
TOXICOLOGICAL PROFILE FOR BARIUM AND BARIUM COMPOUNDS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2007 BARIUM AND BARIUM COMPOUNDS ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. BARIUM AND BARIUM COMPOUNDS iii UPDATE STATEMENT A Toxicological Profile for Barium and Barium Compounds, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 BARIUM AND BARIUM COMPOUNDS iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced.