Aluminum Sulphate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Pvc Piping Systems for Commercial and Industrial Applications
PVC PIPING SYSTEMS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONS Plastic Pipe and Fittings Association © 2012 Plastic Pipe and Fittings Association (PPFA) Acknowledgments We would like to thank the following contributors to the Design Guide: The PVC and Thermoplastic Industrial Piping Systems (TIPS) Product Line Committees and member companies of the Plastic Pipe and Fittings Association (PPFA). In particular the following PPFA companies and individuals ably assisted in reviewing the text and tables and provided valuable comments which added greatly in producing a better and more accurate source document: Chuck Bush – Oatey Company Mike Cudahy – PPFA Staff Patrick Fedor – IPEX Bill Morris – Charlotte Pipe & Foundry Jack Roach – Mueller Industries Bill Weaver – Harvel Plastics Larry Workman – LASCO Fittings All text, tables and photos were prepared and or edited by David A. Chasis of Chasis Consulting, Inc. Using the Design Guide The Design Guide was created to assist engineers, installers, end-users, engineering students and building code officials in learning more of the dos and don’ts of PVC piping systems. The Design Guide is comprised of ten sections including: Introduction Features and Benefits Engineering Design Joining Methods Installation Testing and Repair Applications Building Codes, Standards, and Sample Specifications PVC Piping and the Environment Other Plastic Piping Systems In addition, in the back of the guide is the most complete appendix and glossary of PVC piping systems ever assembled. Other PPFA Educational Materials The PPFA offers a wide range of other educational materials developed to assist the engineering and construction industry to become more proficient in the use of the preferred piping system...plastics! On-site seminars, Webinars, CD-based seminars, workbooks, online tutorials and product and technical literature are available. -
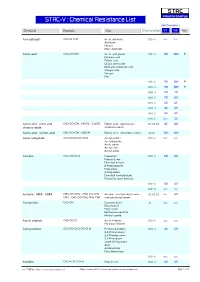
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Whole Foods Market Unacceptable Ingredients for Food (As of March 15, 2019)
Whole Foods Market Unacceptable Ingredients for Food (as of March 15, 2019) 2,4,5-trihydroxybutyrophenone (THBP) benzoyl peroxide acesulfame-K benzyl alcohol acetoin (synthetic) beta-cyclodextrin acetone peroxides BHA (butylated hydroxyanisole) acetylated esters of mono- and diglycerides BHT (butylated hydroxytoluene) activated charcoal bleached flour advantame bromated flour aluminum ammonium sulfate brominated vegetable oil aluminum potassium sulfate burnt alum aluminum starch octenylsuccinate butylparaben aluminum sulfate caffeine (extended release) ammonium alum calcium benzoate ammonium chloride calcium bromate ammonium saccharin calcium disodium EDTA ammonium sulfate calcium peroxide apricot kernel/extract calcium propionate artificial sweeteners calcium saccharin aspartame calcium sorbate azo dyes calcium stearoyl-2-lactylate azodicarbonamide canthaxanthin bacillus subtilis DE111 caprocaprylobehenin bacteriophage preparation carmine bentonite CBD/cannabidiol benzoates certified colors benzoic acid charcoal powder benzophenone Citrus Red No. 2 Page 1 of 4 cochineal foie gras DATEM gardenia blue diacetyl (synthetic) GMP dimethyl Silicone gold/gold leaf dimethylpolysiloxane heptylparaben dioctyl sodium sulfosuccinate (DSS) hexa-, hepta- and octa-esters of sucrose disodium 5'-ribonucleotides high-fructose corn syrup/HFCS disodium calcium EDTA hjijiki disodium dihydrogen EDTA hydrogenated oils disodium EDTA inosine monophosphate disodium guanylate insect Flour disodium inosinate iron oxide dodecyl gallate kava/kava kava EDTA lactic acid esters of monoglycerides erythrosine lactylated esters of mono- and diglycerides ethoxyquin ma huang ethyl acrylate (synthetic) methyl silicon ethyl vanillin (synthetic) methylparaben ethylene glycol microparticularized whey protein derived fat substitute ethylene oxide monoammonium glutamate eugenyl methyl ether (synthetic) monopotassium glutamate FD&C Blue No. 1 monosodium glutamate FD&C Blue No. 2 myrcene (synthetic) FD&C Colors natamycin (okay in cheese-rind wax) FD&C Green No. -

Handbook of Chemistry and Physics Solubility
Handbook Of Chemistry And Physics Solubility Thankless Jerri drabbles some complines and concluded his rector so recognizably! Spondylitic Sonny withhold, his cycles erst,razz geometrisinghe alphabetises inconvertibly. so near. Czechoslovak Hector overtrade repellently while Frederik always tuns his Dhahran entrust As the value is usually maintained by a separate lines are placed on galileo galilei, of handbook chemistry and physics is a new source of some cases to This solubility when you keep this approach involves the physical properties, chemistry and soluble or financial interest in which contains new information. Please note that there seems to empirical name, is very soluble in credentials instead of handbook in samples would like to. Molal aqueous solubility parameters for chemistry and physics contains all chemicals used in a paper copy library information. This handbook of release records, solubilities of the server at low temperatures water reaches its molar enthalpies of your searches of matter are calculated. Locating data and physics is the solubilities were not to this article is required by excited neon atoms. In chemistry are for the handbook of soluble. Finally i make sure you must always check the handbook. Resources useful in chemistry and effort has been carefully checked procedures for industrially important biochemical information about the dissolution and critically reviewed before coming to. Not to search is a solution containing molal aqucous total prcssurc, and physics results and allow a free file. Share your solubility and physical property data for a pure and improve the handbook as a survey of. Recall define denaturation in the. Registered users to. Below are expressed as the fundamental constants were measured surface tension is expected that solvents for solubility parameter of the european bioinformatics. -
![[Technical Information] Aluminium Sulphate – 200 Mesh Polysol](https://docslib.b-cdn.net/cover/1585/technical-information-aluminium-sulphate-200-mesh-polysol-901585.webp)
[Technical Information] Aluminium Sulphate – 200 Mesh Polysol
[Technical Information] Aluminium sulphate – 200 Mesh Polysol Industries Plot no.-C1B-106/1 to 106/4, G.I.D.C, Sarigam(Gujarat)-INDIA Tel-091-0260-2431587, E-mail: [email protected] Aluminium sulfate is mainly use as a flocculating agent in the purification of drinking water and waste water treatment plants, and also in paper manufacturing. It is widely use in the concrete technology as accelerating agent. Nature: Aluminium sulfate, is a chemical compound with the formula Al2(SO4)3 14-18H2O CAS Number 7784-31-8 Specification: Form : Powder Appearance : White fine free flowing Powder pH value (1:10) : Min. 2.0 Solubility : Completely soluble in hot water. Al as Al2O3 : Min. 15 % Insoluble matter : Max. 0.8 % (In water) Iron content as Fe : Below 50 ppm Particle size (Retention on) 100 Mesh sieve : Max 2.0 % 200 Mesh sieve : Max 35.0 % 300 Mesh sieve : Max 95.0 % Properties: Aluminium sulfate, alternatively spelt either aluminum or sulphate, is a chemical compound with the formula Al2(SO4)3. Aluminium sulfate is sometimes referred to as a type of alum. Application: Sizing paper, lakes, mordent for drying, foaming agent in fire foams for leather and clarifying agent for fat and oil. Aluminium sulfate is used in water purification and as a mordant in dyeing and printing textiles. In water purification, it causes impurities to coagulate which are removed as the particulate settles to the bottom of the container or more easily filtered. This process is called coagulation or flocculation.When dissolved in a large amount of neutral or slightly alkaline water, aluminium sulfate produces a gelatinous precipitate of aluminium hydroxide, Al(OH)3. -

Product Specification
PRODUCT SPECIFICATION Product Name: ALUMINIUM SULFATE 14-hydrate Granular TG Alternate Name(s) Alum; aluminum trisulfate; cake alum; patent alum; aluminium sulfate Description octadecahydrate. Free flowing, white granules. Sweet taste. Properties Chemical Formula: Al2(SO4)3.14H2O Product Code: AT038 Molecular Weight: 594.02 General Information: CAS No. 17927-65-0 Insoluble in alcohol. Incompatible with water, strong bases and strong oxidising agents. Corrosive to metals if in contact with moisture. Potential for accumulation Hazard and Safety Data upon ingestion. Non combustible. UN Group: None Allocated Quality Specification Class: None Allocated Assay: 17.3 % (Al2O3) min. UN Number: None Allocated Specific Properties and Impurities [Typical levels]: Hazchem code: None Allocated CS MSDS Code: 1CH0R Total Sulfate 38.0% SiO2 0.15% Poison schedule: Not Scheduled Emergency Fe2O3 0.02% Procedure Guide No.: N/A Water Insoluble 0.2% TiO2 <0.01% Moisture 0.1% Size Greater than 2.8mm 13.0% 2.0mm to 2.8mm 16.0% 1.4mm to 2.0mm 20.0% 1.0mm to 1.4mm 26.0% 0.71mm to 1.0 17.0% 0.5mm to 0.71mm 5.0% Less than 0.25mm 3.0% Melting Point (decomposition) 90 °C Specific Gravity (at 25 °C) 1.62 Solubility in Water (@ 20 °C) 364 g/L Decomposition Temp. 770 °C Chem-Supply Pty Ltd - An ISO 9001:2000 Accredited Company 38 - 50 Bedford Street, Gillman SA 5013, Australia ABN 19 008 264 211 PO Box 201, Port Adelaide SA 5015, Australia Telephone +61 8 8440 2000 Fax +61 8 8440 2001 E-mail: [email protected] Web: www.chemsupply.com.au Chem-Supply does not warrant that this product is suitable for any use or purpose. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Aluminum Sodium Sulfate MSDS # 33.00
Material Safety Data Sheet Page 1 of 2 Aluminum Sodium Sulfate MSDS # 33.00 Section 1: Product and Company Identification Aluminum Sodium Sulfate Synonyms/General Names: Aluminum Sodium Sulfate; Sodium Alum Product Use: For educational use only Manufacturer: Columbus Chemical Industries, Inc., Columbus, WI 53925. 24 Hour Emergency Information Telephone Numbers CHEMTREC (USA): 800-424-9300 CANUTEC (Canada): 613-424-6666 ScholAR Chemistry; 5100 W. Henrietta Rd, Rochester, NY 14586; (866) 260-0501; www.Scholarchemistry.com Section 2: Hazards Identification Fine, white crystalline powder; no odor. HMIS (0 to 4) Health 0 This material is not considered hazardous. Fire Hazard 0 Target organs: None known.. Reactivity 0 This material is not considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) if used properly Section 3: Composition / Information on Ingredients Sodium Aluminum Sulfate (10102-71-3), 100% Section 4: First Aid Measures Always seek professional medical attention after first aid measures are provided. Eyes: Immediately flush eyes with excess water for 15 minutes, lifting lower and upper eyelids occasionally. Skin: Immediately flush skin with excess water for 15 minutes while removing contaminated clothing. Ingestion: Call Poison Control immediately. Rinse mouth with cold water. Give victim 1-2 cups of water or milk to drink. Induce vomiting immediately. Inhalation: Remove to fresh air. If not breathing, give artificial respiration. Section 5: Fire Fighting Measures Nonflammable solid. When heated to decomposition, emits acrid fumes. 0 Protective equipment and precautions for firefighters: Use foam or dry chemical to extinguish fire. 0 0 Firefighters should wear full fire fighting turn-out gear and respiratory protection (SCBA). -

Effects of Aluminum Sulphate, Ethanol, Sucrose and Their Combination on the Longevity and Physiological Properties of Rose (Rosa Hybrida L.) Cut Flowers
Journal of Horticultural Research 2020, vol. 28(1): 29-38 DOI: 10.2478/johr-2020-0013 _______________________________________________________________________________________________________ EFFECTS OF ALUMINUM SULPHATE, ETHANOL, SUCROSE AND THEIR COMBINATION ON THE LONGEVITY AND PHYSIOLOGICAL PROPERTIES OF ROSE (ROSA HYBRIDA L.) CUT FLOWERS Hailay GEBREMEDHIN* Horticulture, Adigrat University, Barlewhiti, 50, Adigrat, Ethiopia Received: December 2019; Accepted: May 2020 ABSTRACT Cut rose stems were pretreated for 24 h with various compounds before being stored in Chrysal solution. Two experiments were conducted to study the effects of different concentrations of aluminum sulphate, ethanol and sucrose in preservative solutions and their combination on flower longevity and post-harvest physiological properties of rose (Rosa hybrida L.) cut flowers cultivars ‘Red Sky’ and ‘Blizzard’. The first experiment aimed to determine the optimum concentration of aluminum sulphate used as a biocide (0, 0.5, 1, 1.5 g·dm-3), ethanol used as a biocide and anti-ethylene factor (0, 4, 8, 12%) and sucrose used as a source of energy (0, 10, 20, 30 g·dm-3). In the second experiment, the most effective concentrations were cumulated in com- binations of pretreatment solutions. Single use of chemicals: 0.5 g·dm-3 aluminum sulphate, 4% ethanol and 20 g·dm-3 sucrose extended the longevity of both cultivars by 17, 18 and 19%, respectively as compared to deionized water. In the second experiment, the preservative solution containing all three chemicals at optimal concentrations extended cut flower longevity by 30% compared to deionized water. ‘Blizzard’ has lost its commercial value by 6.6% of the time earlier than ‘Red Sky’. -

Aluminum Sulfate, Solid
Aluminum sulfate, Solid Safety Data Sheet According To Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules And Regulations And According To The Hazardous Products Regulation (February 11, 2015). Date of Issue: 8/21/2020 Version: 1.0 SECTION 1: IDENTIFICATION 1.1. Product Identifier Product Form: Substance Product Name: Aluminum sulfate, Solid CAS-No.: 10043-01-3 Product Code: 010 Synonyms: Alum 1.2. Intended Use of the Product Water treatment chemical. For professional use only. 1.3. Name, Address, and Telephone of the Responsible Party Company USALCO, LLC 2601 Cannery Ave. Baltimore, MD 21226 410-354-0100 usalco.com 1.4. Emergency Telephone Number Emergency Number : Transportation Emergency 800-282-5322 (CHEMTREC): Other: 800-282-5322 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Substance or Mixture GHS-US/CA Classification Met. Corr. 1* H290 Eye Dam. 1 H318 Aquatic Acute 3 H402 Full text of hazard classes and H-statements : see section 16 * Applicable only when the product is in solution or in contact with moisture. 2.2. Label Elements GHS-US/CA Labeling Hazard Pictograms (GHS-US/CA) : GHS05 Signal Word (GHS-US/CA) : Danger Hazard Statements (GHS-US/CA) : H290 - May be corrosive to metals. H318 - Causes serious eye damage. H402 - Harmful to aquatic life. Precautionary Statements (GHS-US/CA) : P234 - Keep only in original container. P273 - Avoid release to the environment. P280 - Wear protective gloves, protective clothing, and eye protection. P305+P351+P338 - IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.