Introduction to Fertilizers Industries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Department of Chemistry
ST.PHILOMENA’S COLLEGE (AUTONOMOUS), MYSURU (AFFILIATED TO UNIVERSITY OF MYSORE) REACCREDITED BY NAAC WITH A GRADE COURSE – B.Sc Three-year six semesters Choice Based Credit System (CBCS) and Continuous Assessment & Grading Pattern (CAGP) Under Graduate Programme under Autonomous Structure Academic year 2018-19 onwards DEPARTMENT OF CHEMISTRY St. Philomena’s College (Autonomous) Mysuru. B.Sc., Chemistry Syllabus - CBCS Scheme 2018-19 onwards. Page 1 PREAMBLE For the development of any Society, Science education plays an important role. Chemistry, being a major component of Science, is one of the increasingly important disciplinary areas of Science. Chemistry, which is also studied at the Bachelor’s degree programme for years, has been witnessing a slow transition from an analog to a much- needed one. It is an experimental science and students need to be trained both in the theoretical & practical aspects to get expertise. Moreover, the topics prescribed should provide in-depth knowledge of the subject and also the relevant basic allied subjects. Under this context, in order to make the U. G. teaching more effective and meaningful, revamping the syllabus is the need of the hour. It is certain that systematic and planned curricula from first to the third year shall motivate and encourage students for pursuing higher studies in various disciplines of chemistry such as Inorganic, organic, Physical, Analytical and Bio-Chemistry. This curriculum also enables the students to shoulder the responsibility as chemists in chemical industry. Thus, an updated and content revision of UG Chemistry syllabus is essential to improve its quality at the National and International level and also to meet the present-day challenges of PG and research oriented work after the PG programme. -

Ammonia Snow-Lines and Ammonium Salts Desorption ? F
Astronomy & Astrophysics manuscript no. aanda_ammonium-salts ©ESO 2021 April 22, 2021 Ammonia snow-lines and ammonium salts desorption ? F. Kruczkiewicz1; 2; 3, J. Vitorino2, E. Congiu2, P. Theulé1, and F. Dulieu2 1 Aix Marseille Univ, CNRS, CNES, LAM, Marseille, France e-mail: [email protected] 2 CY Cergy Paris Université, Observatoire de Paris, PSL University, Sorbonne Université, CNRS, LERMA, F-95000, Cergy, France 3 Max-Planck-Institut für extraterrestrische Physik, Gießenbachstraße 1, Garching, 85748, Germany Received –; accepted – ABSTRACT Context. The nitrogen reservoir in planetary systems is a long standing problem. Part of the N-bearing molecules is probably incor- porated into the ice bulk during the cold phases of the stellar evolution, and may be gradually released into the gas phase when the ice is heated, such as in active comets. The chemical nature of the N-reservoir should greatly influence how, when and in what form N returns to the gas phase, or is incorporated into the refractory material forming planetary bodies. Aims. We present the study the thermal desorption of two ammonium salts: ammonium formate and ammonium acetate from a gold surface and from a water ice substrate. Methods. Temperature-programmed desorption experiments and Fourier transform infrared reflection spectroscopy were conducted to investigate the desorption behavior of ammonium salts. Results. Ammonium salts are semi-volatile species releasing neutral species as major components upon desorption, that is ammonia and the corresponding organic acid (HCOOH and CH3COOH), at temperatures higher than the temperature of thermal desorption of water ice. Their desorption follows a first-order Wigner-Polanyi law. We find the first order kinetic parameters A = 7.7 ± 0.6 × 1015 s−1 −1 20 −1 −1 and Ebind = 68.9 ± 0.1 kJ mol for ammonium formate and A = 3.0 ± 0.4 × 10 s and Ebind = 83.0 ± 0.2 kJ mol for ammonium acetate. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -
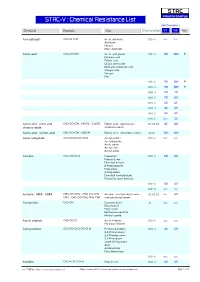
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

New Mineral Names*,†
American Mineralogist, Volume 106, pages 1360–1364, 2021 New Mineral Names*,† Dmitriy I. Belakovskiy1, and Yulia Uvarova2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2CSIRO Mineral Resources, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151, Australia In this issue This New Mineral Names has entries for 11 new species, including 7 minerals of jahnsite group: jahnsite- (NaMnMg), jahnsite-(NaMnMn), jahnsite-(CaMnZn), jahnsite-(MnMnFe), jahnsite-(MnMnMg), jahnsite- (MnMnZn), and whiteite-(MnMnMg); lasnierite, manganflurlite (with a new data for flurlite), tewite, and wumuite. Lasnierite* the LA-ICP-MS analysis, but their concentrations were below detec- B. Rondeau, B. Devouard, D. Jacob, P. Roussel, N. Stephant, C. Boulet, tion limits. The empirical formula is (Ca0.59Sr0.37)Ʃ0.96(Mg1.42Fe0.54)Ʃ1.96 V. Mollé, M. Corre, E. Fritsch, C. Ferraris, and G.C. Parodi (2019) Al0.87(P2.99Si0.01)Ʃ3.00(O11.41F0.59)Ʃ12 based on 12 (O+F) pfu. The strongest lines of the calculated powder X-ray diffraction pattern are [dcalc Å (I%calc; Lasnierite, (Ca,Sr)(Mg,Fe)2Al(PO4)3, a new phosphate accompany- ing lazulite from Mt. Ibity, Madagascar: an example of structural hkl)]: 4.421 (83; 040), 3.802 (63, 131), 3.706 (100; 022), 3.305 (99; 141), characterization from dynamic refinement of precession electron 2.890 (90; 211), 2.781 (69; 221), 2.772 (67; 061), 2.601 (97; 023). It diffraction data on submicrometer sample. European Journal of was not possible to perform powder nor single-crystal X-ray diffraction Mineralogy, 31(2), 379–388. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -
![An African Green Revolution, Finding Ways to Boost Productivity on Small Farms {Keijiro Otsuka} [940075759X] (2012).Pdf](https://docslib.b-cdn.net/cover/1924/an-african-green-revolution-finding-ways-to-boost-productivity-on-small-farms-keijiro-otsuka-940075759x-2012-pdf-941924.webp)
An African Green Revolution, Finding Ways to Boost Productivity on Small Farms {Keijiro Otsuka} [940075759X] (2012).Pdf
Keijiro Otsuka Donald F. Larson Editors An African Green Revolution Finding Ways to Boost Productivity on Small Farms An African Green Revolution Keijiro Otsuka • Donald F. Larson Editors An African Green Revolution Finding Ways to Boost Productivity on Small Farms Editors Keijiro Otsuka Donald F. Larson National Graduate Institute The World Bank for Policy Studies Research Group 7-2 2-1 Roppongi 1818 H Street Northwest Minato , Tokyo 106-8677 Washington , DC 20433 Japan USA The fi ndings, interpretations, and conclusions expressed in this work do not necessarily re fl ect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. ISBN 978-94-007-5759-2 ISBN 978-94-007-5760-8 (eBook) DOI 10.1007/978-94-007-5760-8 Springer Dordrecht Heidelberg New York London Library of Congress Control Number: 2012951120 © The International Bank for Reconstruction and Development/The World Bank 2013 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, speci fi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on micro fi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. -

Flight Through the Comet Chury's Dust Cloud Resolves Chemical Mystery 21 January 2020
Flight through the comet Chury's dust cloud resolves chemical mystery 21 January 2020 em. Hans Balsiger, was on board. A key finding from the measurements taken by this instrument was that there appeared to be a lack of nitrogen in Halley's coma—the nebulous covering of comets which forms when a comet passes close to the sun. Although nitrogen (N) was discovered in the form of ammonia (NH3) and hydrocyanic acid (HCN), the incidence was far removed from the expected cosmic incidence. More than 30 years later, researchers have solved this mystery thanks to a happy accident. This is a result of the analysis of data from the Bernese mass spectrometer ROSINA, which collected data on the comet 67P/Churyumov-Gerasimenko, called Chury for short, on board the ESA space probe Rosetta (see info box below). Risky flight through the comet Chury's dust cloud Less than a month before the end of the Rosetta A plume of dust from Comet mission, the space probe was just 1.9 km above 67P/Churyumov–Gerasimenko, seen by the OSIRIS Wide Angle Camera on ESA's Rosetta spacecraft on 3 the surface of Chury as it flew through a dust cloud July 2016. The shadow of the plume is cast across the from the comet. This resulted in a direct impact of basin, which is in the Imhotep region. Credit: dust in the ion source of the mass spectrometer ESA/Rosetta/MPS for OSIRIS Team ROSINA-DFMS (Rosetta Orbiter Sensor for Ion and MPS/UPD/LAM/IAA/SSO/INTA/UPM/DASP/IDA Neutral Analysis-Double Focusing Mass Spectrometer), led by the University of Bern. -

Fertilizer Use in African Agriculture Lessons Learned and Good Practice Guidelines Public Disclosure Authorized Michael Morris,Valerie A
Public Disclosure Authorized DIRECTIONS IN DEVELOPMENT 39037 Public Disclosure Authorized Agriculture and Rural Development Fertilizer Use in African Agriculture Lessons Learned and Good Practice Guidelines Public Disclosure Authorized Michael Morris,Valerie A. Kelly, Ron J. Kopicki, and Derek Byerlee Public Disclosure Authorized Fertilizer Use in African Agriculture Fertilizer Use in African Agriculture Lessons Learned and Good Practice Guidelines Michael Morris Valerie A. Kelly Ron J. Kopicki Derek Byerlee © 2007 The International Bank for Reconstruction and Development / The World Bank 1818 H Street, NW Washington, DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org E-mail: [email protected] All rights reserved. 1 2 3 4 :: 10 09 08 07 This volume is a product of the staff of the International Bank for Reconstruction and Development / The World Bank. The findings, interpretations, and conclusions expressed in this volume do not necessarily reflect the views of the Executive Directors of The World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Rights and Permissions The material in this publication is copyrighted. Copying and/or transmitting portions or all of this work without permission may be a violation of applicable law. The International Bank for Reconstruction and Development / The World Bank encourages dissemination of its work and will normally grant permission to reproduce portions of the work promptly.