(12) Patent Application Publication (10) Pub. No.: US 2009/0133788 A1 Mungas Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (1O) Patent No.: �US 8,572,946 B2 Mungas Et Al
11111111111111111111111111111111111111111111111111111111111111111111111111 (12) United States Patent (1o) Patent No.: US 8,572,946 B2 Mungas et al. (45) Date of Patent: Nov. 5, 2013 (54) MICROFLUIDIC FLAME BARRIER 1,103,503 A 7/1914 Goddard 1,586,195 A 5/1926 Hall (75) Inventors: Gregory S. Mungas, Mojave, CA (US); 2,609,281 A 9/1952 Smith 2,714,563 A 2/1955 Norman et al. David J. Fisher, Tehachapi, CA (US); 3,243,272 A3/1966 Schmitz Christopher Mungas, Plymouth, CA 3,266,241 A * 8/1966 Jennings ......................... 60/258 (US) 3,512,556 A 5/1970 McKhann 3,719,046 A 3/1973 Sutherland et al. (73) Assignee: Firestar Engineering, LLC, Mojave, 3,779,714 A 12/1973 Nadkarni et al. CA (US) (Continued) (*) Notice: Subject to any disclaimer, the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. EP 1500880 1/2005 EP 1500880 A2 1/2005 (21) Appl. No.: 13/549,027 (Continued) (22) Filed: Jul. 13, 2012 OTHER PUBLICATIONS "aRocket", an amateur rocketry discussion forum onhttp://exrocktry . (65) Prior Publication Data net/mailman/listinfo/arocket, Dec. 31, 2009. US 2012/0279196 Al Nov. 8, 2012 (Continued) Related U.S. Application Data Primary Examiner Phutthiwat Wongwian (63) Continuation-in-part of application No. 11/950,174, filed on Dec. 4, 2007, now Pat. No. 8,230,672, and a (74) Attorney, Agent, or Firm HolzerIPLaw, PC continuation of application No. 12/613,188, filed on Nov. 5, 2009, now Pat. No. 8,230,673. (57) ABSTRACT (60) Provisional application No. -

The NASA In-Space Propulsion Technology Project's Current
NASA In-Space Propulsion Technologies and Their Infusion Potential David J. Anderson,1 Eric J. Pencil,2 Todd Peterson3 and Daniel Vento4 NASA Glenn Research Center, Cleveland, OH 44135 Michelle M. Munk5 and Louis J. Glaab6 NASA Langley Research Center, Hampton, VA 23681 and John W. Dankanich7 AeroDank, Inc., Cleveland, OH 44135 The In-Space Propulsion Technology (ISPT) program has been developing in-space propulsion technologies that will enable or enhance NASA robotic science missions. The ISPT program is currently developing technology in four areas that include Propulsion System Technologies (Electric and Chemical), Entry Vehicle Technologies (Aerocapture and Earth entry vehicles), Spacecraft Bus and Sample Return Propulsion Technologies (components and ascent vehicles), and Systems/Mission Analysis. Three technologies are ready for flight infusion: 1) the high-temperature Advanced Material Bipropellant Rocket (AMBR) engine providing higher performance; 2) NASA’s Evolutionary Xenon Thruster (NEXT) ion propulsion system, a 0.6-7 kW throttle-able gridded ion system; and 3) Aerocapture technology development with investments in a family of thermal protection system (TPS) materials and structures; guidance, navigation, and control (GN&C) models of blunt-body rigid aeroshells; and aerothermal effect models. Two component technologies that will be ready for flight infusion in the near future will be Advanced Xenon Flow Control System, and ultra-lightweight propellant tank technologies. Future focuses for ISPT are sample return missions and other spacecraft bus technologies like: 1) Mars Ascent Vehicles (MAV); 2) multi-mission technologies for Earth Entry Vehicles (MMEEV) for sample return missions; and 3) electric propulsion for sample return and low cost missions. -

An Analysis of Green Propulsion Applied to NASA Missions
An Analysis of Green Propulsion Applied to NASA Missions Presented at Space Propulsion 2014 Cologne, GERMANY May 19-22nd, 2014 Eric H. Cardiff1, Henry W. Mulkey2, and Caitlin E. Bacha3, NASA Goddard Space Flight Center, Greenbelt, MD 20771 The advantages of green propulsion for five mission classes are examined, including a Low Earth Orbit (LEO) mission (GPM), a Geosynchronous Earth Orbit (GEO) mission (SDO), a High Earth Orbit (HEO) mission (MMS), a lunar mission (LRO), and a planetary mission (MAVEN). The propellant mass benefits are considered for all five missions, as well as the effects on the tanks, propellant loading, thruster throughput, thermal considerations, and range requirements for both the AF-M315E and LMP-103S propellants. Nomenclature ACS = Attitude Control System MMH = Monomethyl Hydrazine CDR = Critical Design Review MMS = Magnetospheric Multi Scale mission ECAPS = Ecological Advanced Propulsion NASA = National Aeronautics and Space Systems Administration GEO = Geosynchronous Orbit NOFBX = Nitrous Oxide Fuel Blend GSFC = Goddard Space Flight Center NTO = Nitrogen Tetroxide GPIM = Green Propellant Infusion Mission PRISMA = Prototype Research Instruments and GPM = Global Precipitation Measurement Space Mission Advancement HEO = High Earth Orbit RE = Earth Radii IA = International Agreement SCAPE = Self-Contained Atmospheric Protective Isp = Specific Impulse (s) Ensemble LEO = Low Earth Orbit SDO = Solar Dynamics Observatory LRO = Lunar Reconnaissance Orbiter SNSB = Swedish National Space Board mf = Spacecraft final (or dry) mass STMD = Space Technology Mission Directorate mi = Spacecraft initial (or wet) mass TDRSS = Tracking and Data Relay Satellite MAVEN = Mars Atmosphere and Volatile System Evolution mission TRL = Technology Readiness Level ME = Main Engine (for SDO) I. Introduction Interest in green propulsion has rekindled in recent years due to the ultimate success of catalytic ignition systems for multiple high-performance formulations. -

Electric Feed Systems for Liquid-Propellant Rockets
Research Report. ELECTRIC FEED SYSTEMS FOR LIQUID PROPELLANT ROCKET ENGINES. Pablo Rachov, Hernán Tacca y Diego Lentini. Cita: Pablo Rachov, Hernán Tacca y Diego Lentini (2010). ELECTRIC FEED SYSTEMS FOR LIQUID PROPELLANT ROCKET ENGINES. Research Report. Dirección estable: https://www.aacademica.org/hernan.emilio.tacca/9 Esta obra está bajo una licencia de Creative Commons. Para ver una copia de esta licencia, visite http://creativecommons.org/licenses/by-nc-nd/4.0/deed.es. Acta Académica es un proyecto académico sin fines de lucro enmarcado en la iniciativa de acceso abierto. Acta Académica fue creado para facilitar a investigadores de todo el mundo el compartir su producción académica. Para crear un perfil gratuitamente o acceder a otros trabajos visite: http://www.aacademica.org. RESEARCH REPORTS ELECTRIC FEED SYSTEMS FOR LIQUID PROPELLANT ROCKET ENGINES Pablo RACHOV LABORATORIO DE CONTROL DE ACCIONAMIENTOS, TRACCIÓN Y POTENCIA (LABCATYP) Departamento de Electrónica Facultad de Ingeniería UNIVERSIDAD DE BUENOS AIRES Directors: Prof. Hernán TACCA, University of Buenos Aires, Argentina Prof. Diego LENTINI, University of Roma “La Sapienza”, Italy Buenos Aires, December 6th, 2010. DOI: 10.13140/2.1.4431.9042 Comparison of Liquid Propellant Rocket Engine Feed Systems - 1 - 1 REPORT N o 1 COMPARISON OF LIQUID PROPELLANT ROCKET ENGINE FEED SYSTEMS Pablo RACHOV LABORATORIO DE CONTROL DE ACCIONAMIENTOS, TRACCIÓN Y POTENCIA (LABCATYP) Departamento de Electrónica Facultad de Ingeniería UNIVERSIDAD DE BUENOS AIRES Directors: Prof. Hernán TACCA, University of Buenos Aires, Argentine Prof. Diego LENTINI, University of Roma “La Sapienza”, Italy Buenos Aires, December 6th, 2010. Comparison of Liquid Propellant Rocket Engine Feed Systems - 1 - 2 CONTENTS I. -

Introduction to Fertilizers Industries
Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org ADANA UNIVERSTY – INDUSTRY JOINT RESEARCH CENTER INTRODUCTIN TO FERTILIZER INDUSTRIES BY TAREK ISMAIL KAKHIA 1 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org ADANA UNIVERSTY – INDUSTRY JOINT RESEARCH CENTER page Item 3 Fertilizer 71 N - P - K rating 17 Fertilizers Inorganic Acids: 21 Sulfur 53 Sulfur Dioxide 11 Sulfur Trioxide 44 Sulfuric Acid 35 Nitrogen 35 Liquid Nitrogen 37 Nitrogen Cycle 51 Nitrogen Oxide 53 Nitric oxide ( NOX ) 22 Nitrogen Di Oxide 27 Nitrous Oxide 111 Nitric Oxide 121 Di Nitrogen Pent oxide 714 Nitric Acid 151 Phosphate Minerals ( Phosphate Rock (Phospharite ٌ 157 155 Phosphorus 132 Phosphorus Oxide 171 Phosphorus Tri Oxide 172 Phosphorus Pent Oxide 711 Phosphoric Acid 137 Phospho Gypsum 711 Fertilizers Alkalizes: 787 Ammonia 211 Ammonia Production 211 Amine Gas Treating 171 Ammonium Hydroxide 212 Category : Ammonium Compounds 221 Potassium 253 Potassium Hydroxide 211 Fertilizers Salts 215 Ammonium Ferric Citrate 2 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org ADANA UNIVERSTY – INDUSTRY JOINT RESEARCH CENTER 211 Ammonium Nitrate 215 Di Ammonium Phosphate 212 Tri Ammonium Phosphate 231 Ammonium Sulfate 232 Calcium Nitrate 231 Calcium Phosphate 233 Mono Calcium Phosphate 271 Di Calcium Phosphate 271 Tri Calcium Phosphate 271 Sodium Nitrate 267 Magnesium Phosphate 275 Di Magnesium Phosphate 272 Magnesium Sulfate 235 Potassium Chloride 235 Potassium Citrate 251 Potassium Nitrate 253 Potassium Phosphate 257 Mono Potassium Phosphate 255 Di Potassium Phosphate 252 Tri Potassium Phosphate 221 Potassium Sulfate 221 Borax 511 Organic Fertilizers: 515 Compost 513 Composting 521 Urea 555 Urea Cycle 555 Urea Phosphate 331 Extension & Supplements 511 Macronutrient & Micronutrient Fertilizers 535 Category : Phosphate minerals 533 Pozzolan 535 Pumice 5 Copyright © Tarek Kakhia. -

Propulsion Systems Design • Rocket Engine Basics • Survey of the Technologies • Propellant Feed Systems • Propulsion Systems Design
Propulsion Systems Design • Rocket engine basics • Survey of the technologies • Propellant feed systems • Propulsion systems design © 2016 David L. Akin - All rights reserved http://spacecraft.ssl.umd.edu U N I V E R S I T Y O F Rocket Propulsion MARYLAND 1 ENAE 791 - Launch and Entry Vehicle Design Liquid Rocket Engine Cutaway U N I V E R S I T Y O F Rocket Propulsion MARYLAND 2 ENAE 791 - Launch and Entry Vehicle Design Thermal Rocket Exhaust Velocity • Exhaust velocity is ! + γ −1. 2γ ℜT - % p ( γ 0 ! 0 ' e * Ve = -1 −' * 0 γ −1 M - & p ) 0 ! , 0 / where M ≡ average molecular weight of exhaust Joules ℜ ≡ universal gas const.= 8314.3 mole°K γ ≡ ratio of specific heats ≈1.2 U N I V E R S I T Y O F Rocket Propulsion MARYLAND 3 ENAE 791 - Launch and Entry Vehicle Design €€ € € Ideal Thermal Rocket Exhaust Velocity • Ideal exhaust velocity is ! 2γ ℜT V = 0 ! e γ −1 M • Tis corresponds to an ideally expanded nozzle • All thermal energy converted to kinetic energy of exhaust • Only a function of temperature and molecular weight! U N I V E R S I T Y O F Rocket Propulsion MARYLAND 4 ENAE 791 - Launch and Entry Vehicle Design € Thermal Rocket Performance • Trust is ! T = m˙ Ve + ( pe − pamb)Ae • Effective exhaust velocity ! A " c % T m˙ c c V p p e $ I = ' ! = ⇒ = e + ( e − amb) $ sp ' m˙ # g0 & • Expansion ratio 1 1 * γ −1- γ −1# & γ # & γ At # γ +1& pe γ +1, pe / = % ( % ( ,1 −% ( / A $ 2 ' $ p ' γ −1, $ p ' / e 0 + 0 . -

Excea Dolum Cum Audaerionsed Que Exerum Ni Bea Vel Issint Eaqui
ISSN 1742-5689 | Volume 12 | Issue 102 | 6 January 2015 J. R. Soc. Interface | R. Soc. Interface J. The Royal Society is a self-governing Fellowship of many of the world’s most distinguished scientists 12 Volume drawn from all areas of science, engineering, and medicine. The Society’s fundamental purpose, as it has been since its foundation in 1660, is to recognise, | promote, and support excellence in science and to Issue 102 encourage the development and use of science for Excea dolum cum audaerionsed Puda entiberro et acerchit quibus Sum vel intiberume nonsequam the benefi t of humanity. que exerum ni bea vel issint eaqui et molupta nostis eriorrumquis experci tatquiatias et quid rerupta ipic totaquiat doluptibu. eium, simporitas maximen moluptatur arunt doluptibus quibus | The Society’s strategic priorities emphasise its 6 January 2015 commitment to the highest quality science, to doluptibu diaerendi re magnisquist, net occus est, curiosity-driven research, and to the development and use of science for the benefi t of society. These priorities are: • Promoting science and its benefi ts • Recognising excellence in science • Supporting outstanding science • Providing scientifi c advice for policy • Fostering international and global cooperation • Education and public engagement For further information on the Royal Society The Royal Society 6 – 9 Carlton House Terrace London SW1Y 5AG T +44 20 7451 2500 W royalsociety.org For further information on Journal of the Royal Society Interface T +44 20 7451 2633 E [email protected] W rsif.royalsocietypublishing.org 1742-5689(201501)12:102 ISSN 1742-5689 The Royal Society Registered Charity No 207043 SUBSCRIPTIONS In 2015 J. -

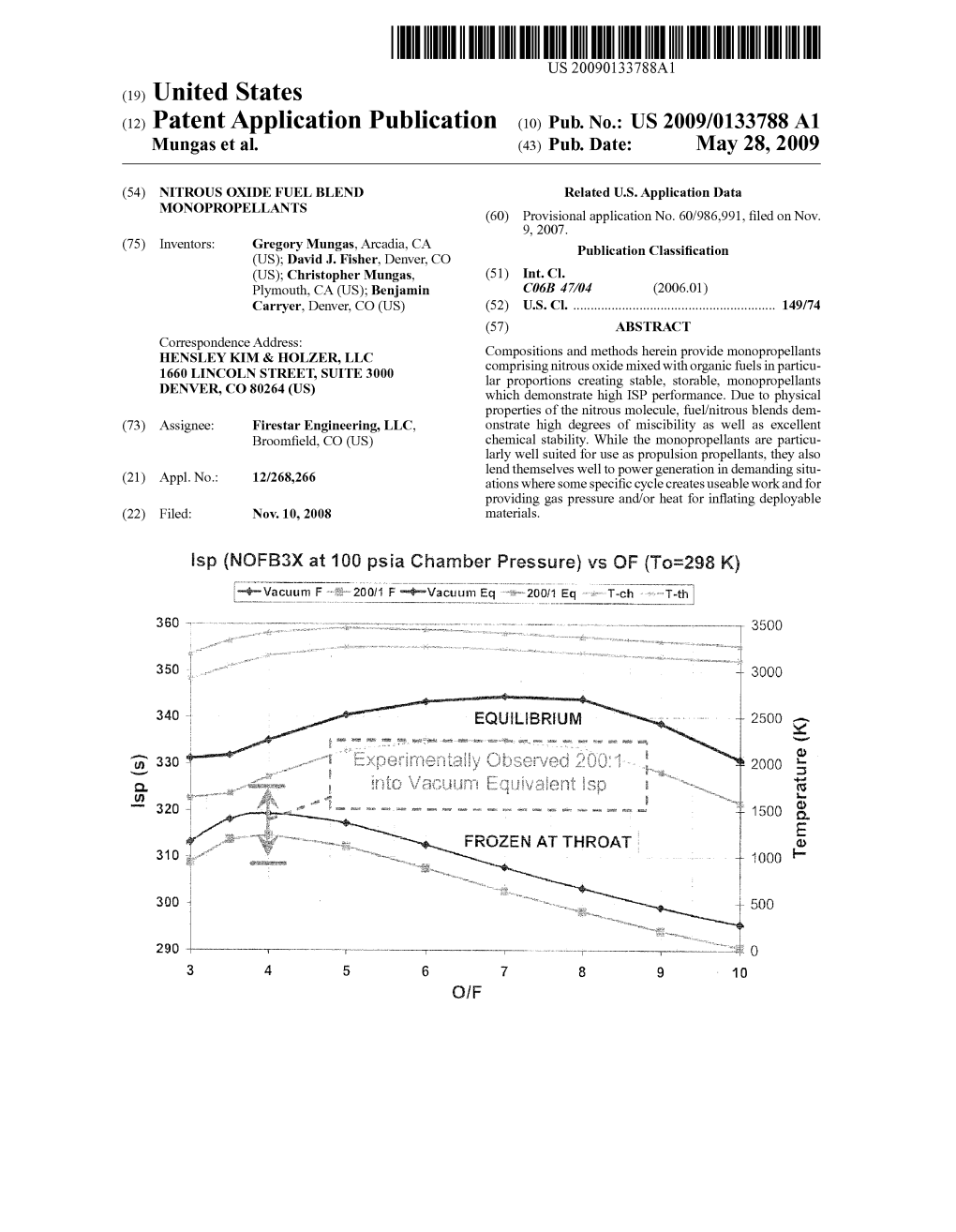
Nofbx™ Mars Ascent Vehicle: a Single Stage to Orbit Approach J
Concepts and Approaches for Mars Exploration (2012) 4353.pdf NOFBX™ MARS ASCENT VEHICLE: A SINGLE STAGE TO ORBIT APPROACH J. M. Vozoff1, D. J. Fisher2, and G. S. Mungas3, 1mv2space, 2Firestar Technologies, 3 Firestar Technologies, 1122 Flight Line Street #76, Mojave, CA 93501, [email protected]. Introduction: Nitrous Oxide Fuel Blend ing TRL 6, having successfully begun testing in a vac- (NOFBX™) is a nitrous-oxide-based mono-propulsion uum test facility. technology developed specifically for a Mars Ascent NOFBX™ technology is more than the propellants Vehicle (MAV) application by Firestar Technologies. themselves: it is also the essential surrounding tech- This development was funded primarily by the NASA nologies that enable this propellant to be safely and Mars Program Office, NASA Small Business Innova- effectively employed to generate thrust in spacecraft tion Research (SBIR), and NASA International Space and rocket propulsion applications. Some examples Station (ISS). NOFBX™ propulsion system specific are flashback arrestor technologies, porous media in- impulse performance equals or exceeds state-of-the-art jectorhead technology, microfluidic regererative thrust nitrogen textroxide/ monomethyl hydrazine chamber technology and the associated thin-film fabri- (NTO/MMH) systems, with the added advantages of cation technique, Firestar’s developments to-date have restartability via spark ignition, deep throttleability demonstrated practical application of the technology (100:1), and high thrust-to-weight ratio. For a Mars -

Chemistry 1 Stokiometry Contents
chemistry 1 stokiometry Contents 1 John Dalton 1 1.1 Early life ................................................ 1 1.2 Early careers .............................................. 1 1.3 Scientific contributions ........................................ 1 1.3.1 Meteorology ......................................... 1 1.3.2 Colour blindness ....................................... 1 1.3.3 Measuring mountains in the Lake District .......................... 2 1.3.4 Gas laws ............................................ 2 1.3.5 Atomic theory ......................................... 2 1.3.6 Atomic weights ........................................ 3 1.3.7 Other investigations ...................................... 3 1.3.8 Experimental approach .................................... 4 1.4 Other publications ........................................... 4 1.5 Public life ............................................... 4 1.6 Personal life .............................................. 5 1.7 Disability and death .......................................... 5 1.8 Legacy ................................................. 5 1.9 See also ................................................ 6 1.10 References ............................................... 6 1.11 Sources ................................................ 7 1.12 External links ............................................. 8 2 Atomic theory 9 2.1 History ................................................. 9 2.1.1 Philosophical atomism .................................... 9 2.1.2 Dalton ............................................ -

Review of State-Of-The-Art Green Monopropellants: for Propulsion Systems Analysts and Designers
aerospace Review Review of State-of-the-Art Green Monopropellants: For Propulsion Systems Analysts and Designers Ahmed E. S. Nosseir 1,2,*, Angelo Cervone 1,* and Angelo Pasini 2 1 Department of Space Engineering, Faculty of Aerospace Engineering, Delft University of Technology (TU Delft), 2629 HS Delft, The Netherlands 2 Sede di Ingegneria Aerospaziale, Dipartimento di Ingegneria Civile e Industriale, Università di Pisa (UniPi), 56122 Pisa, Italy; [email protected] * Correspondence: [email protected] or [email protected] (A.E.S.N.); [email protected] (A.C.) Abstract: Current research trends have advanced the use of “green propellants” on a wide scale for spacecraft in various space missions; mainly for environmental sustainability and safety concerns. Small satellites, particularly micro and nanosatellites, evolved from passive planetary-orbiting to being able to perform active orbital operations that may require high-thrust impulsive capabilities. Thus, onboard primary and auxiliary propulsion systems capable of performing such orbital op- erations are required. Novelty in primary propulsion systems design calls for specific attention to miniaturization, which can be achieved, along the above-mentioned orbital transfer capabilities, by utilizing green monopropellants due to their relative high performance together with simplicity, and better storability when compared to gaseous and bi-propellants, especially for miniaturized systems. Owing to the ongoing rapid research activities in the green-propulsion field, it was necessary to extensively study and collect various data of green monopropellants properties and performance that would further assist analysts and designers in the research and development of liquid propulsion sys- tems. -

Research on Future Orbital Propulsion Systems and Scramjet Engines
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Institute of Transport Research:Publications 70th International Astronautical Congress, Washington, USA. Copyright 2019 by German Aerospace Center (DLR). Published by the IAF, with permission and released to the IAF to publish in all forms. IAC-19,C4,9,1,x49899 TEST COMPLEX M11: RESEARCH ON FUTURE ORBITAL PROPULSION SYSTEMS AND SCRAMJET ENGINES Wilhelm, M.*, Werling, L., Strauss, F., Lauck, F., Kirchberger, C., Ciezki, H., Schlechtriem, S. German Aerospace Center (DLR), Institute of Space Propulsion, Hardthausen, Germany * Corresponding Author: [email protected] Basic research activities and thrust chamber oriented technology development for rocket and air-breathing (Ram-/SCRamjet) propulsion systems are conducted at Test Complex M11. History of M11 dates back to the year 1966, when the first building was constructed. Since its existence, lots of scientific results could be elaborated in the multifarious working areas and have been published in a large number of publications in journals and on conferences. Today’s research and development activities are focused on combustion, ignition, cooling and chamber processes of advanced green propellants for rockets, satellites and orbital propulsion systems. Furthermore material testing as well as propellant synthesis and analysis are performed. This publication gives a short overview of the history of the test complex and delivers an inside into current research activities. 1. HISTORICAL ACTIVITIES [1] (STudentenRaketeN). Fig. 2 shows the current view of Test Complex M11 at DLR site Lampoldshausen the M11 with the student test field M11.5 on the left was built in the 60s of the 20th century. -

Masten Flight Systems Works Toward First Launch of a NASA Payload For
The National Aeronautics and Space Administration Desert Thunder! Masten Flight Systems works toward first launch of a NASA payload for Flight Opportunities office Cover Story Welcome to Aerovations Flight Opportunities This is an exciting time within NASA for innovation Resources 16 The newly established Office of the and technology. With the Chief Technologist seeks to match NASA Ronald Young, creation of the NASA Office Dryden Innovative and science community launch needs of the Chief Technologist, Partnerships Office with companies capable of getting the and the selection of its chief, 661-276-3741 experiments aloft to flight conditions Bobby Braun, a new agency champion has emerged The Office of the Chief to re-vitalize NASA as a Technologist: http://www. preeminent research and 30 Holland helps nasa.gov/offices/oct/home/ 24 Fiber optic development organization. index.html News protect ideas, Expanding on initiatives 4 Dryden Center systems commercialize tech of the former Innovative SBIR and STTR Innovation Fund Partnerships Program, information: http://sbir. new models are nurturing gsfc.nasa.gov/SBIR/SBIR. selectees named 6 Allen Parker’s ED11 0115-05 NASA Photo by Tom Tschida Concepts innovations inside and html 11 Dryden researcher algorithm shatters a outside of NASA. In fact, The Innovative Partnerships Office staff includes, from left, Jason Han- technology barrier as part of the OCT, all of son, Ron Young, Julie Holland, Greg Poteat and Elizabeth Newcamp. submits winning idea for 9 New launch Dryden IPP website: the IPP – now called the http://www.nasa. office is formulated from two previous projects. Ames Research future space treadmills technology idea 6 Dryden, 4DSP enter Innovative Partnerships gov/centers/dryden/ Center, Moffett Field, Calif., began the Commercial Reusable gains ground Office – has been adopted Suborbital Research, or CRuSR, activity for the agency’s Science OCT structure is licensing agreement about/Organizations/ 17 and its work continued.