Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Pdf 462.69 K
Journal of Oil, Gas and Petrochemical Technology Vol. 5, No. 1 , pp. 63 - 75 6 3 The Investigation of Purity Improvement for the Production of Methyl Propionate in Different Types of Batch Distillation Systems Dhia Y. Aqar * 1 , Hassan H. Al Alak 2 , N. Rahmanian 3 , I.M. Mujtaba 3 1 Ministry of Oil, South Refineries Company, Basra, Iraq 2 Department of Materials Engineering, Faculty of Engineering, University of Kufa, Najaf, Iraq 3 Faculty of Engineering and Informatics, University of Bradford, Bradford, UK ARTICLE INFO ABSTRACT Received: January 06, 2018 Methyl propionate, also known as methyl propanoate, Accepted: September 08, 2018 is a clear colourless liquid with a characteristic odour (fruity smel l and taste). In this study , the formation of methyl propionate through the esterification o Keywords : propionic acid and methanol was inves tigated in the Methyl propionate reactive distillation system using a conventional (CBD), CBD single feed (SF - SBD), and double feed semi - batch SF - SBD distillatio n (DF - SBD) columns for the first time . The Double feed semi - batch distillation performances measure o f these distillation systems (DF - SBD) were evaluated in terms of min imum batch time for a given separation task. The optimization results clearly * Corresponding author: show ed that the DF - SBD system is a more attractive E - mail: : [email protected] operation in terms of reaction conversion, and maximum achievable purity as compared to the SF SBD, and CBD processes. 64 Dhia Y. Aqar et al. 1. Introduction Methyl propionate is a very important component which has useful applications in a variety of areas in the chemical industry such as solvents for cellulose nitrate, lacquers, plasticizers, chemical intermediates, fragrances, flavors, a raw material in organic synthesis for the production of varnishes, paints, and other chemical compound s such as methyl methacrylate [1] . -

R Graphics Output
Dexamethasone sodium phosphate ( 0.339 ) Melengestrol acetate ( 0.282 ) 17beta−Trenbolone ( 0.252 ) 17alpha−Estradiol ( 0.24 ) 17alpha−Hydroxyprogesterone ( 0.238 ) Triamcinolone ( 0.233 ) Zearalenone ( 0.216 ) CP−634384 ( 0.21 ) 17alpha−Ethinylestradiol ( 0.203 ) Raloxifene hydrochloride ( 0.203 ) Volinanserin ( 0.2 ) Tiratricol ( 0.197 ) trans−Retinoic acid ( 0.192 ) Chlorpromazine hydrochloride ( 0.191 ) PharmaGSID_47315 ( 0.185 ) Apigenin ( 0.183 ) Diethylstilbestrol ( 0.178 ) 4−Dodecylphenol ( 0.161 ) 2,2',6,6'−Tetrachlorobisphenol A ( 0.156 ) o,p'−DDD ( 0.155 ) Progesterone ( 0.152 ) 4−Hydroxytamoxifen ( 0.151 ) SSR150106 ( 0.149 ) Equilin ( 0.3 ) 3,5,3'−Triiodothyronine ( 0.256 ) 17−Methyltestosterone ( 0.242 ) 17beta−Estradiol ( 0.24 ) 5alpha−Dihydrotestosterone ( 0.235 ) Mifepristone ( 0.218 ) Norethindrone ( 0.214 ) Spironolactone ( 0.204 ) Farglitazar ( 0.203 ) Testosterone propionate ( 0.202 ) meso−Hexestrol ( 0.199 ) Mestranol ( 0.196 ) Estriol ( 0.191 ) 2,2',4,4'−Tetrahydroxybenzophenone ( 0.185 ) 3,3,5,5−Tetraiodothyroacetic acid ( 0.183 ) Norgestrel ( 0.181 ) Cyproterone acetate ( 0.164 ) GSK232420A ( 0.161 ) N−Dodecanoyl−N−methylglycine ( 0.155 ) Pentachloroanisole ( 0.154 ) HPTE ( 0.151 ) Biochanin A ( 0.15 ) Dehydroepiandrosterone ( 0.149 ) PharmaCode_333941 ( 0.148 ) Prednisone ( 0.146 ) Nordihydroguaiaretic acid ( 0.145 ) p,p'−DDD ( 0.144 ) Diphenhydramine hydrochloride ( 0.142 ) Forskolin ( 0.141 ) Perfluorooctanoic acid ( 0.14 ) Oleyl sarcosine ( 0.139 ) Cyclohexylphenylketone ( 0.138 ) Pirinixic acid ( 0.137 ) -

11 Carboxylic Acids and Derivatives
CARBOXYLIC ACIDS AND NITRILES Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 CARBOXYLIC ACID DERIVATIVES 2 CARBOXYLIC ACID DERIVATIVES R C N nitrile R = CH3 acetonitrile 3 STRUCTURE AND BONDING 4 NOMENCLATURE—THE IUPAC SYSTEM heptanoic acid benzoic acid cyclopentane carboxylic acid 4,5-dimethyl 3-pentenoic 3-bromobenzoic p-toluic 1-methyl- hexanoic acid acid acid acid cyclopropanecarboxylic acid 5 NOMENCLATURE-COMMON NAMES προτοσ πιον 6 NOMENCLATURE-POLYIACIDS Oxalis acetosella Malon Succinum HOOC COOH COOH propane-1,2,3-tricarboxylic acid 7 PHYSICAL PROPERTIES • Carboxylic acids exhibit dipole-dipole interactions because they have polar C—O and O—H bonds. • They also exhibit intermolecular hydrogen bonding. • In the gas phase and in apolar solvents, carboxylic acids often exist as dimers held together by two intermolecular hydrogen bonds. 8 PHYSICAL PROPERTIES 9 ACIDITY OF CARBOXYLIC ACIDS The acetate anion has two C—O bonds of equal length (1.27 Å) and intermediate between the length of a C—O single bond (1.36 Å) and C=O (1.21 Å). 10 CARBOXYLIC ACIDS—STRONG ORGANIC BRØNSTED-LOWRY ACIDS 11 THE INDUCTIVE EFFECT IN ALIPHATIC CARBOXYLIC ACIDS 12 THE INDUCTIVE EFFECT IN ALIPHATIC CARBOXYLIC ACIDS 13 SUBSTITUTED BENZOIC ACIDS 14 SUBSTITUTED BENZOIC ACIDS 15 PREPARATION OF CARBOXYLIC ACIDS [1] Oxidation of 1° alcohols [2] Oxidation of alkyl benzenes 16 PREPARATION OF CARBOXYLIC ACIDS [3] From alkyl halides CN CN- i. NaOH + ii.H3O Br COOH i. CO2 Mg + ii. H3O MgBr R MgBr R OMgBr H O+ R OH 3 + Mg++ + Br- O C O O O 17 PREPARATION OF CARBOXYLIC ACIDS [5] From alkyl halides. -

Experimental and Modeling Study of the Thermal Decomposition of Methyl Decanoate Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc
Experimental and modeling study of the thermal decomposition of methyl decanoate Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc To cite this version: Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc. Experimental and modeling study of the thermal decomposition of methyl decanoate. Combustion and Flame, Elsevier, 2011, 158 (7), pp.1288-1300. 10.1016/j.combustflame.2010.11.009. hal-00724939 HAL Id: hal-00724939 https://hal.archives-ouvertes.fr/hal-00724939 Submitted on 23 Aug 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Experimental and modeling study of the thermal decomposition of methyl decanoate Olivier Herbineta,*, Pierre-Alexandre Glaudea, Valérie Wartha and Frédérique Battin-Leclerca a Laboratoire Réactions et Génie des Procédés, Nancy Université, CNRS UPR 3349, BP 20451, 1 rue Grandville, 54001 Nancy, France Abstract The experimental study of the thermal decomposition of methyl decanoate was performed in a jet-stirred reactor at temperatures ranging from 773 to 1123 K, at residence times between 1 and 4 s, at a pressure of 800 Torr (106.6 kPa) and at high dilution in helium (fuel inlet mole fraction of 0.0218). -

Amine-Catalyzed Hydrolyses of Cyclodextrin Cinnamates
Proc. Natl. Acad. Sci. USA Vol. 73, No. 9, pp. 2969-2972, September 1976 Chemistry Amine-catalyzed hydrolyses of cyclodextrin cinnamates (cyclodextrin-catalyzed ester hydrolysis/acyl-cyclodextrin/acceleration of deacylation/enzyme model) MAKOTO KOMIYAMA AND MYRON L. BENDER Division of Biochemistry, Department of Chemistry, Northwestern University, Evanston, Illinois 60201 Contributed by Myron L. Bender, July 6,1976 ABSTRACT Hydrolyses of 6B-cyclodextrin cinnamate EXPERIMENTAL (#CDC) and a-cyclodextrin cinnamate were catalyzed by amines such as 1,4-diazabicyclo(2.2.2)octane, triethylamine, quinucli- Materials. #CDC and aCDC were kindly furnished by Y. dine, piperidine, diisobutylamine, and n-butylamine. The rate constant of hydrolyses of the #CDC-amine complexes follows Kurono. They were recrystallized before use. The purity of the order: 1,4-diazabicyclo(2.2.2)octane > n-butylamine > qui- fCDC and aCDC were found to be higher than 96% and-98%, nuclidine > piperidine > triethylamine >> diisobutylamine. The respectively, by absorption spectroscopy at 273 nm, which is ratio of the catalytic rate constant for the ,8CDC/1,4-diazabi- the isosbestic point between trans-cinnamic acid and (3CDC cyclo(2.2.2)octane complex to the spontaneous rate constant for or aCDC. Aza2bicOct was purified by recrystallization and had ,8CDC is about 6-fold and is almost independent of pH below a melting point of 158° see ref. Other pH 11.5; but, it then drastically increases with pH above pH 11.5, (159-160° reported, 5). up to 57-fold at pH 13.6 which is much higer than previous reagents were purchased from the Aldrich Chemical Co. attempts. -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -

Hydrolysis of Amides: a Kinetic Study of Substituent Effects on the Acidic and Basic Hydrolysis of Aliphatic Amides
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 1969 Hydrolysis of amides: a kinetic study of substituent effects on the acidic and basic hydrolysis of aliphatic amides Grahame Leslie Jackson Wollongong University College Follow this and additional works at: https://ro.uow.edu.au/theses University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Jackson, Grahame Leslie, Hydrolysis of amides: a kinetic study of substituent effects on the acidic and basic hydrolysis of aliphatic amides, Doctor of Philosophy thesis, Department of Chemistry, University of Wollongong, 1969. -
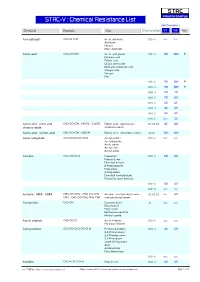
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Expanding the Modular Ester Fermentative Pathways for Combinatorial Biosynthesis of Esters from Volatile Organic Acids
ARTICLE Expanding the Modular Ester Fermentative Pathways for Combinatorial Biosynthesis of Esters From Volatile Organic Acids Donovan S. Layton,1,2 Cong T. Trinh1,2,3 1 Department of Chemical and Biomolecular Engineering, University of Tennessee, Knoxville, Tennessee 2 BioEnergy Science Center (BESC), Oak Ridge National Laboratory, Oak Ridge, Tennessee 3 Bredesen Center for Interdisciplinary Research and Graduate Education, University of Tennessee, Knoxville, Tennessee; telephone: þ865-974-8121; fax: 865-974-7076; e-mail: [email protected] Biotechnol. Bioeng. 2016;113: 1764–1776. ABSTRACT: Volatile organic acids are byproducts of fermentative ß 2016 Wiley Periodicals, Inc. metabolism, for example, anaerobic digestion of lignocellulosic KEYWORDS: modular chassis cell; carboxylate; ester; acyl acetate; biomass or organic wastes, and are often times undesired inhibiting acyl acylate; ester fermentative pathway cell growth and reducing directed formation of the desired products. Here, we devised a general framework for upgrading these volatile organic acids to high-value esters that can be used as flavors, fragrances, solvents, and biofuels. This framework employs the acid-to-ester modules, consisting of an AAT (alcohol Introduction acyltransferase) plus ACT (acyl CoA transferase) submodule and an alcohol submodule, for co-fermentation of sugars and organic Harnessing renewable or waste feedstocks (e.g., switchgrass, corn acids to acyl CoAs and alcohols to form a combinatorial library of stover, agricultural residue, or municipal solid waste) -

Assessment of Free and Immobilized Kefir Culture in Simultaneous
LWT - Food Science and Technology 76 (2017) 67e78 Contents lists available at ScienceDirect LWT - Food Science and Technology journal homepage: www.elsevier.com/locate/lwt Assessment of free and immobilized kefir culture in simultaneous alcoholic and malolactic cider fermentations Anastasios Nikolaou a, Alex Galanis a, Maria Kanellaki b, Chrysoula Tassou c, * Konstantoula Akrida-Demertzi d, Yiannis Kourkoutas a, a Laboratory of Applied Microbiology and Biotechnology, Department of Molecular Biology & Genetics, Democritus University of Thrace, Alexandroupolis, GR-68100, Greece b Food Biotechnology Group, Section of Analytical Environmental and Applied Chemistry, Department of Chemistry, University of Patras, Patras, GR-26500, Greece c Institute of Technology of Agricultural Products, Hellenic Agricultural Organization DEMETER, 1 S. Venizelou Str, Lykovrissi, Athens, GR-14123, Greece d Laboratory of Food Chemistry and Technology, Department of Chemistry, University of Ioannina, Dourouti, Ioannina, GR-45110, Greece article info abstract Article history: The aim of the present study was to assess application of free or immobilized kefir culture on apple Received 30 March 2016 pieces and delignified cellulosic material (DCM) in simultaneous alcoholic and malolactic cider fer- Received in revised form mentations at a wide temperature range (5e45 C). Repeated batch fermentations were continued for 12 October 2016 higher than 7 months, showing a high operational stability of the systems and were completed in less Accepted 13 October 2016 than 24 h with immobilized cells on DCM at 37 C. Malic acid conversion up to 71.5% and ethanol Available online 15 October 2016 productivity values up to 56.9 g/(Ld) were recorded, which could be adopted by the industrial sector.