Food and Drug Administration, HHS § 172.515
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -
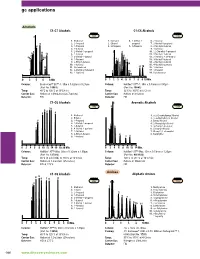
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Visible-Light-Driven Photooxidation of Alcohols Using Surface-Doped Graphitic Carbon Nitride
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2017 Visible-Light-Driven Photooxidation of alcohols using surface-doped graphitic carbon nitride Wuyuan Zhang, Anna Bariotaki, Ioulia Smonou, and Frank Hollmann* Material and Methods Materials Unless stated otherwise all chemicals were purchased from Sigma-Aldrich, Fluka, Acros or Alfa-Aesar with the highest purity available and used without further treatment. Synthesis of g-C3N4 and carbon nanodots doped (CD-C3N4) The g-C3N4 was synthesized via the simple calcination (Carbolite furnace; CWF 12/5, 2400 W) of urea (10.0 g) at 600 °C for 4 h (5 °C/min), a yellowish powder was obtained after the cooling to room temperature.1 The carbon nanodots were synthesized via the thermal decomposition of sucrose with slight modification.2 Briefly, 0.75 g of sucrose was dissolved in 30 mL of MilliQ water and stirred at room temperature for 0.5 h. The solution was transferred into a 45 mL Teflon-lined stainless steel autoclave reactor, and heated at 180 °C for 5 h (10 °C/min). After cooling the reactor to room temperature, a brown mixture was obtained. The mixture was centrifuged (8000 rpm for 20 min), and the pellet was washed with water (3×) and freeze-dried. 3 In order to deposit carbon nanodots to the surface of g-C3N4, Liu et al’s procedures were adopted. Firstly, a stock solution of carbon nanodots (1 mg in 25mL water) was prepared. 15.0 mL of this stock solution was mixed with 15.0 mL of NH4OH (28 %) and sealed in a 45 mL Teflon-lined autoclave reactor. -

(12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry Et Al
US00757617OB2 (12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry et al. (45) Date of Patent: Aug. 18, 2009 (54) CYCLIC SILOXANE COMPOSITIONS FOR 6,046,156 A 4/2000 Perry THE RELEASE OF ACTIVE INGREDIENTS 6,054,547 A 4/2000 Perry et al. 6,063,365 A 5, 2000 Schefer et al. (75) Inventors: Robert J. Perry, Niskayuna, NY (US); 6,075,111 A 6/2000 Perry et al. Mark D. Leatherman, Elmsford, NY 6,077.923 A 6/2000 Perry et al. (US); Shahid Murtuza, Albany, NY 6,083,901 A 7/2000 Perry et al. (US) 6,121,343 A 9/2000 Hongo et al. 6,143,309 A 11/2000 Legrow et al. (73) Assignee: Momentive Performance Materials, 6,153,578 A 11/2000 Perry Albany, NY (US) 6,200,949 B1 3/2001 Reijmer et al. 6,228,380 B1 5, 2001 LeGrow et al. (*) Notice: Subject to any disclaimer, the term of this 6,262,287 B1 7/2001 Anderson et al. patent is extended or adjusted under 35 6,267,977 B1 7/2001 LeGrow et al. U.S.C. 154(b) by 993 days. 6,309,715 B1 10/2001 Lindauer et al. 6,322,777 B1 1 1/2001 Perry et al. (21) Appl. No.: 10/742,415 6,325,274 B2 12/2001 Esumi et al. 6,325,859 B1 12/2001 De Roos et al. (22) Filed: Dec. 19, 2003 6,435,423 B2 8/2002 Hurry et al. (65) Prior Publication Data 6,624,136 B2 9, 2003 Guerinet al. -

Akshi Bansal* B. K. Dangarh ABSTRACT KEYWORDS
ORIGINAL RESEARCH PAPER Volume-7 | Issue-1 | January-2018 | PRINT ISSN No 2277 - 8179 INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH OXIDATION STUDY OF 4-METHOXYBENZYL ALCOHOL BY CR (VI) OXIDANTS IN PARTIAL AQUEOUS MEDIUM- A COMPARATIVE STUDY Chemistry Research Scholar, Pacific academy of Higher Education and Research University, Akshi Bansal* Udaipur *Corresponding Author B. K. Dangarh Asstt. Prof. of Chemistry, Govt. P. G. College, Neemuch, M.P. ABSTRACT Oxidation of 4-Methoxybenzyl alcohol by different Cr (VI) oxidants [PDC and PCC] has been studied in acetic acid-H2O medium in the presence of PTSA spectrophotometrically. The reactions are found first order with respect to both the oxidants, [H+], and [substrate].Michaelis-Menten type kinetics is observed. The reaction rate decreases with increasing volume percentage of acetic acid in reaction mixture. The reaction was studied at different temperature [298-318 K] and activation parameters were computed. The oxidation product was identified as Cr (III) and corresponding aldehyde. The oxidation rate order was found with respect to the oxidants are: PCC > PDC KEYWORDS Oxidation, 4-Methoxybenzyl alcohol, PTSA, PCC and PDC. 1. INTRODUCTION The kinetics run were followed for more than 60-70% completion of 4-Methoxybenzyl alcohol (Anisyl alcohol) is used as a fragrance and the reaction and good first order kinetics were observed. flavouring. It occurs naturally but is produced by reduction of anisaldehyde. 3. RESULTS AND DISCUSSION: 3.1 Stoichiometry and product analysis: Kinetics and mechanism of the oxidation of substituted benzyl To determine the stoichiometry of a reaction a known slight excess of alcohols by pyridinium chlorochromate studied by Banerji[1] et al. -

Pdf 462.69 K
Journal of Oil, Gas and Petrochemical Technology Vol. 5, No. 1 , pp. 63 - 75 6 3 The Investigation of Purity Improvement for the Production of Methyl Propionate in Different Types of Batch Distillation Systems Dhia Y. Aqar * 1 , Hassan H. Al Alak 2 , N. Rahmanian 3 , I.M. Mujtaba 3 1 Ministry of Oil, South Refineries Company, Basra, Iraq 2 Department of Materials Engineering, Faculty of Engineering, University of Kufa, Najaf, Iraq 3 Faculty of Engineering and Informatics, University of Bradford, Bradford, UK ARTICLE INFO ABSTRACT Received: January 06, 2018 Methyl propionate, also known as methyl propanoate, Accepted: September 08, 2018 is a clear colourless liquid with a characteristic odour (fruity smel l and taste). In this study , the formation of methyl propionate through the esterification o Keywords : propionic acid and methanol was inves tigated in the Methyl propionate reactive distillation system using a conventional (CBD), CBD single feed (SF - SBD), and double feed semi - batch SF - SBD distillatio n (DF - SBD) columns for the first time . The Double feed semi - batch distillation performances measure o f these distillation systems (DF - SBD) were evaluated in terms of min imum batch time for a given separation task. The optimization results clearly * Corresponding author: show ed that the DF - SBD system is a more attractive E - mail: : [email protected] operation in terms of reaction conversion, and maximum achievable purity as compared to the SF SBD, and CBD processes. 64 Dhia Y. Aqar et al. 1. Introduction Methyl propionate is a very important component which has useful applications in a variety of areas in the chemical industry such as solvents for cellulose nitrate, lacquers, plasticizers, chemical intermediates, fragrances, flavors, a raw material in organic synthesis for the production of varnishes, paints, and other chemical compound s such as methyl methacrylate [1] . -

Experimental and Modeling Study of the Thermal Decomposition of Methyl Decanoate Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc
Experimental and modeling study of the thermal decomposition of methyl decanoate Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc To cite this version: Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc. Experimental and modeling study of the thermal decomposition of methyl decanoate. Combustion and Flame, Elsevier, 2011, 158 (7), pp.1288-1300. 10.1016/j.combustflame.2010.11.009. hal-00724939 HAL Id: hal-00724939 https://hal.archives-ouvertes.fr/hal-00724939 Submitted on 23 Aug 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Experimental and modeling study of the thermal decomposition of methyl decanoate Olivier Herbineta,*, Pierre-Alexandre Glaudea, Valérie Wartha and Frédérique Battin-Leclerca a Laboratoire Réactions et Génie des Procédés, Nancy Université, CNRS UPR 3349, BP 20451, 1 rue Grandville, 54001 Nancy, France Abstract The experimental study of the thermal decomposition of methyl decanoate was performed in a jet-stirred reactor at temperatures ranging from 773 to 1123 K, at residence times between 1 and 4 s, at a pressure of 800 Torr (106.6 kPa) and at high dilution in helium (fuel inlet mole fraction of 0.0218). -

A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing A-Cyano Or A-Ester Functional Group from Baylis-Hillman Adducts
Notes Bull. Korean Chem. Soc. 2004, Vol. 25, No. 3 413 A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing a-Cyano or a-Ester Functional Group from Baylis-Hillman Adducts Ka Young Lee, Saravanan GowriSankar, and Jae Nyoung Kim Department of Chemistry and Institute of Basic Science, Chonnam National University, Gwangju 500-757, Korea Received December 18, 2003 Key Words : Cinnamyl alcohols, Baylis-Hillman adducts, Sulfuric acid, Acetic anhydride Cinnamyl alcohols bearing ester, acid, or nitrile functional Hillman alcohol into the primary acetate or eventually to group at the 2-position are very useful synthons for the primary alcohol (Scheme 1 and Table 1). synthesis of various biologically important compounds.1 The reaction of Baylis-Hillman adducts with acetic Synthesis of cinnamyl alcohol derivatives from the Baylis- anhydride (1.5 equiv.) in the presence of sulfuric acid (10 Hillman adducts has been reported by us and other groups.1-4 mol%) gave the corresponding rearranged cinnamyl acetates The conversion can be carried out directly by using 20% quantitatively in dichloromethane at room temperature aqueous sulfuric acid (for the Baylis-Hillman adducts within 30 min (Actually, the cinnamyl acetate derivatives derived from acrylonitrile)2 or trifluoroacetic acid (for the could be isolated in 92-95% yields.). In order to obtain the Baylis-Hillman adducts derived from acrylates).3 Recently, corresponding cinnamyl alcohols in a one-pot we tried some Basavaiah and coworkers have reported the two-step, one- reaction conditions for the next partial hydrolysis. Addition pot conversion method: TMSOTf-assisted conversion of of water to the reaction mixture (two-phase system) afforded Baylis-Hillman alcohol into the corresponding primary the hydrolyzed cinnamyl alcohols in trace amounts. -

European Patent Office of Opposition to That Patent, in Accordance with the Implementing Regulations
(19) TZZ __T (11) EP 2 416 766 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/045 (2006.01) A61K 45/06 (2006.01) 09.04.2014 Bulletin 2014/15 A61K 8/34 (2006.01) A61P 17/00 (2006.01) A61P 17/16 (2006.01) A61Q 19/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09701247.0 A23L 1/30 A23L 2/02 A23L 2/39 (2006.01) A23C 9/13 (2006.01) A23C 11/10 (2006.01) A61Q 5/02 (2006.01) (22) Date of filing: 09.04.2009 A61Q 11/00 (2006.01) A61Q 15/00 (2006.01) A61Q 17/04 (2006.01) A61Q 19/02 (2006.01) A61K 8/02 (2006.01) A61Q 19/04 (2006.01) (86) International application number: PCT/EP2009/054336 (87) International publication number: WO 2009/087242 (16.07.2009 Gazette 2009/29) (54) COMPOSITIONS COMPRISING TRANS-TERT-BUTYL CYCLOHEXANOL AS SKIN IRRITATION- REDUCING AGENT ZUSAMMENSETZUNGEN MIT TRANS-TERT-BUTYL-CYCLOHEXANOL ALS MITTEL ZUR REDUZIERUNG VON HAUTREIZUNGEN COMPOSITIONS COMPRENANT DU TRANS-TERT-BUTYL CYCLOHEXANOL EN TANT QU’AGENT RÉDUISANT L’IRRITATION CUTANÉE (84) Designated Contracting States: • KROHN, Michael AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 64653 Lorsch (DE) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • ZINKE, Holger PT RO SE SI SK TR 64646 Heppenheim (DE) (43) Date of publication of application: (74) Representative: Eisenführ Speiser 15.02.2012 Bulletin 2012/07 Patentanwälte Rechtsanwälte PartGmbB Postfach 10 60 78 (73) Proprietor: Symrise AG 28060 Bremen (DE) 37603 Holzminden (DE) (56) References cited: (72) Inventors: EP-A2- 0 755 910 WO-A1-97/22332 • VIELHABER, Gabriele WO-A1-2008/117254 US-A- 2 927 127 75008 Paris (FR) • OERTLING, Heiko • SYMRISE GMBH & CO KG ET AL: "Trans-tert- 1012 Lausanne (CH) butyl cyclohexanol as skin irritation-reducing • GÖMANN, Claudia agent" RESEARCH DISCLOSURE, MASON 37640 Warbsen (DE) PUBLICATIONS, HAMPSHIRE, GB, vol. -

Nomination Background: Methyl Trans
' 7/?1 Methyl styryl ketone 122-57-6 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Nomination Source: NCI 2. Recommendations: -Comparative toxicity studies with methyl vinyl ketone -Metabolism -Carcinogenicity 3. Rationale/Remarks: -Potential for widespread human exposure -Nominated as a result of a class study of a,~-unsaturated ketones -Flavoring and fragrance additive -Natural product -Environmental pollutant -Mutagenic in Salmonella 4. Priority: -High for toxicity studies -Moderate for carcinogenicity s. Date of Nomination: July 1994 B. Interagency Committee for Chemical Evaluation and Coordination Review 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: 122-57-6 Methyl styryl ketone SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Number: 122-57-6 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl- (SCI, 9CI) Svnonvms and Trade Names: Acetocinnamone; benzalacetone; benzylideneacetone; methyl 2 phenylvinyl ketone; methyl styryl ketone; methyl ~-styryl ketone; 4 phenylbutenone; 4-phenyl-3-butene-2-one; 2-phenylvinyl methyl ketone; styryl methyl ketone; MSK FEMA Number: 2881 Related isomers CAS Registry Number: 937-53-1 Chemica] Abstracts Name: 3-Buten-2-one, 4-phenyl-, (Z)- (SCI, 9CI) Synonvms and Trade Names: cis -4-Phenyl-3-buten-2-one; cis -benzalacetone; cis benzylideneacetone; cis-methyl styryl ketone CAS Registry Number: 1896-62-4 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl-, (E)- (SCI, 9CI) Synonyms and Trade Names: trans-4-Phenyl-3-buten-2-one; trans-benzalacetone; trans benzylideneacetone; trans-methyl styryl ketone; TPBO Structure. Molecular Formula. and Molecular WeiKbt 0 II H•CHCCH3 Mol. wt.: 146.19 Chemical and Phvsical Properties (from Aldrich Chemical Co., 1993, 1994; Budavari, 1989) Description: White to yellow solid with a sweet, pungent, creamy, floral odor Prepared for NCI by Technical Resources, Inc. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Functional Expression of a Novel Methanol-Stable Esterase From
Cai et al. BMC Biotechnology (2020) 20:36 https://doi.org/10.1186/s12896-020-00622-1 RESEARCH ARTICLE Open Access Functional expression of a novel methanol- stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent- free system Xianghai Cai1†, Lin Lin2,3†, Yaling Shen1, Wei Wei1* and Dong-zhi Wei1 Abstract Background: Esterases are widely distributed in nature and have important applications in medical, industrial and physiological. Recently, the increased demand for flavor esters has prompted the search of catalysts like lipases and esterases. Esterases from thermophiles also show thermal stability at elevated temperatures and have become enzymes of special interest in biotechnological applications. Although most of esterases catalyzed reactions are carried out in toxic and inflammable organic solvents, the solvent-free system owning many advantages such as low cost and easy downstream processing. Results: The gene estGSU753 from Geobacillus subterraneus DSM13552 was cloned, sequenced and overexpressed into Escherichia coli BL21 (DE3). The novel gene has an open reading frame of 753 bp and encodes 250-amino-acid esterase (EstGSU753). The sequence analysis showed that the protein contains a catalytic triad formed by Ser97, Asp196 and His226, and the Ser of the active site is located in the conserved motif Gly95-X-Ser97-X-Gly99 included in most esterases and lipases. The protein catalyzed the hydrolysis of pNP-esters of different acyl chain lengths, and the enzyme specific activity was 70 U/mg with the optimum substrate pNP-caprylate. The optimum pH and temperature of the recombinant enzyme were 8.0 and 60 °C respectively.