Cigarette Additives, Carcinogens and Chemicals Nicotine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Visible-Light-Driven Photooxidation of Alcohols Using Surface-Doped Graphitic Carbon Nitride
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2017 Visible-Light-Driven Photooxidation of alcohols using surface-doped graphitic carbon nitride Wuyuan Zhang, Anna Bariotaki, Ioulia Smonou, and Frank Hollmann* Material and Methods Materials Unless stated otherwise all chemicals were purchased from Sigma-Aldrich, Fluka, Acros or Alfa-Aesar with the highest purity available and used without further treatment. Synthesis of g-C3N4 and carbon nanodots doped (CD-C3N4) The g-C3N4 was synthesized via the simple calcination (Carbolite furnace; CWF 12/5, 2400 W) of urea (10.0 g) at 600 °C for 4 h (5 °C/min), a yellowish powder was obtained after the cooling to room temperature.1 The carbon nanodots were synthesized via the thermal decomposition of sucrose with slight modification.2 Briefly, 0.75 g of sucrose was dissolved in 30 mL of MilliQ water and stirred at room temperature for 0.5 h. The solution was transferred into a 45 mL Teflon-lined stainless steel autoclave reactor, and heated at 180 °C for 5 h (10 °C/min). After cooling the reactor to room temperature, a brown mixture was obtained. The mixture was centrifuged (8000 rpm for 20 min), and the pellet was washed with water (3×) and freeze-dried. 3 In order to deposit carbon nanodots to the surface of g-C3N4, Liu et al’s procedures were adopted. Firstly, a stock solution of carbon nanodots (1 mg in 25mL water) was prepared. 15.0 mL of this stock solution was mixed with 15.0 mL of NH4OH (28 %) and sealed in a 45 mL Teflon-lined autoclave reactor. -

Electrophysiological and Behavioral Characterization Of
Deletre et al. Parasites & Vectors (2015) 8:316 DOI 10.1186/s13071-015-0934-y RESEARCH Open Access Electrophysiological and behavioral characterization of bioactive compounds of the Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum and Cinnamomum zeylanicum essential oils against Anopheles gambiae and prospects for their use as bednet treatments Emilie Deletre1* , Fabrice Chandre2, Livy Williams3, Claire Duménil1, Chantal Menut4 and Thibaud Martin1,5 Abstract Background: Laboratory and field studies showed that repellent, irritant and toxic actions of common public health insecticides reduce human-vector contact and thereby interrupt disease transmission. One of the more effective strategies to reduce disease risk involves the use of long-lasting treated bednets. However, development of insecticide resistance in mosquito populations makes it imperative to find alternatives to these insecticides. Our previous study identified four essential oils as alternatives to pyrethroids: Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum, Cinnamomum zeylanicum. The objectives of this study were to identify active compounds of these essential oils, to characterize their biological activity, and to examine their potential as a treatment for bednets. Methods: We evaluated the electrophysiological, behavioural (repellency, irritancy) and toxic effects of the major compounds of these oils against Anopheles gambiae strain ‘Kisumu’. Results: Aldehydes elicited the strongest responses and monoterpenes the weakest responses in electroantennogram (EAG) trials. However, EAG responses did not correlate consistently with results of behavioral assays. In behavioral and toxicity studies, several of the single compounds did exhibit repellency, irritancy or toxicity in An. gambiae; however, the activity of essential oils did not always correlate with activity expected from the major components. On the contrary, the biological activity of essential oils appeared complex, suggesting interactions between individual compounds and the insect under study. -

Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness
Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness Level, in Different Cultivars and During Eating Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Gulsah Ozcan Graduate Program in Food Science and Technology The Ohio State University 2010 Thesis Committee: Sheryl Ann Barringer, Adviser W. James Harper John Litchfield 1 Copyright by Gülşah Özcan 2010 ii ABSTRACT Strawberry samples with enzyme activity and without enzyme activity (stannous chloride added) were measured for real time formation of lipoxygenase (LOX) derived aroma compounds after 5 min pureeing using selected ion flow tube mass spectrometry (SIFT-MS). The concentration of (Z)-3-hexenal and (E)-2-hexenal increased immediately after blending and gradually decreased over time while hexanal concentration increased for at least 5 min in ground strawberries. The formation of hexanal was slower than the formation of (Z)-3-hexenal and (E)-2-hexenal in the headspace of pureed strawberries. The concentration of LOX aldehydes and esters significantly increased during refrigerated storage. Damaging strawberries increased the concentration of LOX aldehydes but did not significantly affect the concentration of esters. The concentrations of many of the esters were strongly correlated to their corresponded acids and/or aldehydes. The concentration of LOX generated aldehydes decreased during ripening, while fruity esters increased. Different varieties had different aroma profiles and esters were the greatest percentage of the volatiles. The aroma release of some of the LOX derived aldehydes in the mouthspace in whole strawberries compared to chopped strawberries showed that these volatiles are formed in the mouth during chewing. -

Next-Generation Sequencing of Representational Difference Analysis
Planta DOI 10.1007/s00425-017-2657-0 ORIGINAL ARTICLE Next-generation sequencing of representational difference analysis products for identification of genes involved in diosgenin biosynthesis in fenugreek (Trigonella foenum-graecum) 1 1 1 1 Joanna Ciura • Magdalena Szeliga • Michalina Grzesik • Mirosław Tyrka Received: 19 August 2016 / Accepted: 30 January 2017 Ó The Author(s) 2017. This article is published with open access at Springerlink.com Abstract Within the transcripts related to sterol and steroidal Main conclusion Representational difference analysis saponin biosynthesis, we discovered novel candidate of cDNA was performed and differential products were genes of diosgenin biosynthesis and validated their sequenced and annotated. Candidate genes involved in expression using quantitative RT-PCR analysis. Based on biosynthesis of diosgenin in fenugreek were identified. these findings, we supported the idea that diosgenin is Detailed mechanism of diosgenin synthesis was biosynthesized from cycloartenol via cholesterol. This is proposed. the first report on the next-generation sequencing of cDNA-RDA products. Analysis of the transcriptomes Fenugreek (Trigonella foenum-graecum L.) is a valuable enriched in low copy sequences contributed substantially medicinal and crop plant. It belongs to Fabaceae family to our understanding of the biochemical pathways of and has a unique potential to synthesize valuable steroidal steroid synthesis in fenugreek. saponins, e.g., diosgenin. Elicitation (methyl jasmonate) and precursor feeding (cholesterol and squalene) were Keywords Diosgenin Á Next-generation sequencing Á used to enhance the content of sterols and steroidal Phytosterols Á Representational difference analysis of sapogenins in in vitro grown plants for representational cDNA Á Steroidal saponins Á Transcriptome user-friendly difference analysis of cDNA (cDNA-RDA). -

Why Some Migration Conditions for Plastics Are Not Appropriate for Other Fcms
Why some migration conditions for plastics are not appropriate for other FCMs. Peter Oldring Representing 18 associations directly or indirectly involved in FCMs FIP Network 25th May 2016 Parma 1 Extract from Executive Summary (p9) European Parliament report on 1935/2004 (May 2016) ‘As a general trend, stakeholders who are in favour of further EU level harmonisation recommend that EU specific measures should establish a single standard for analytical (testing) methods, such as composition determination, migration testing, risk assessment, but also specific methods for compliance enforcement, thus ensuring that the relevant FCM is tested by companies and competent authorities across the EU with one and the same method. Furthermore, the EU single standard for analytical (testing) methods should be specific for each FCM, thus reflecting its unique properties and avoiding situations where non-harmonised FCMs are tested with methods developed for harmonised FCMs, which could lead to misleading and debatable test results’ 2 DISCLAIMER . As chair of the 18 associations representing non- plastics, I will try and represent them. The initiative started with CEPE, EMPAC and almost immediately CES silicones joined. Other associations have joined since then. However, I have to understand and work with analytical data, in order to make decisions about and determine the safety of my company’s products. The work is embryonic, associations are still joining and the final format will certainly be different to that initially envisaged. 3 Associations participating in Initiative . ACE Beverage cartons – paper, plastics and aluminium flexible . APEAL Steel for rigid metal packaging . CEFIC-FCA Substance suppliers . CELIEGE Cork . CEPE Coatings for rigid metal packaging . -
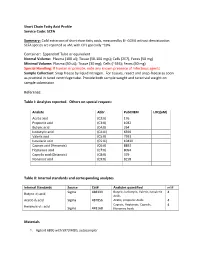
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Method Development and Validation of Vitamin D2 and Vitamin D3 Using Mass Spectrometry
Method Development and Validation of Vitamin D2 and Vitamin D3 Using Mass Spectrometry Devon Victoria Riley A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science University of Washington 2016 Committee: Andrew Hoofnagle Geoffrey Baird Dina Greene Program Authorized to Offer Degree: Laboratory Medicine ©Copyright 2016 Devon V. Riley ii University of Washington Abstract Method Development and Validation of Vitamin D2 and Vitamin D3 Using Mass Spectrometry Devon V. Riley Chair of the Supervisory Committee: Associate Professor Andrew Hoofnagle, MD, PhD Vitamin D has long been known to maintain bone health by regulating calcium and phosphorous homeostasis. In recent years, scientists have discovered additional physiological roles for vitamin D. The complex interaction between the active vitamin D hormone and its metabolic precursors continues to be a rich area of research. Fundamental to this research is the availability of accurate and precise assays. Few published assays for vitamins D2 and D3 have contained sufficient details on method validation or performance characteristics. The liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay developed for this thesis has undergone a rigorous validation and proven to yield a sensitive and specific method that exceeds the capabilities of all previously published methods. Developing and validating a novel assay is often complicated by the lack of established acceptability standards. This thesis explores this challenge, specifically for establishing meaningful interpretations and qualification standards of the lower limit of the measuring interval. Altogether, future research focused on vitamins D2, D3 and the Vitamin D pathway can benefit from this robust LC-MS/MS assay and the associated quality parameters outlined in this thesis. -

US EPA Inert (Other) Pesticide Ingredients
U.S. Environmental Protection Agency Office of Pesticide Programs List of Inert Pesticide Ingredients List 3 - Inerts of unknown toxicity - By Chemical Name UpdatedAugust 2004 Inert Ingredients Ordered Alphabetically by Chemical Name - List 3 Updated August 2004 CAS PREFIX NAME List No. 6798-76-1 Abietic acid, zinc salt 3 14351-66-7 Abietic acids, sodium salts 3 123-86-4 Acetic acid, butyl ester 3 108419-35-8 Acetic acid, C11-14 branched, alkyl ester 3 90438-79-2 Acetic acid, C6-8-branched alkyl esters 3 108419-32-5 Acetic acid, C7-9 branched, alkyl ester C8-rich 3 2016-56-0 Acetic acid, dodecylamine salt 3 110-19-0 Acetic acid, isobutyl ester 3 141-97-9 Acetoacetic acid, ethyl ester 3 93-08-3 2'- Acetonaphthone 3 67-64-1 Acetone 3 828-00-2 6- Acetoxy-2,4-dimethyl-m-dioxane 3 32388-55-9 Acetyl cedrene 3 1506-02-1 6- Acetyl-1,1,2,4,4,7-hexamethyl tetralin 3 21145-77-7 Acetyl-1,1,3,4,4,6-hexamethyltetralin 3 61788-48-5 Acetylated lanolin 3 74-86-2 Acetylene 3 141754-64-5 Acrylic acid, isopropanol telomer, ammonium salt 3 25136-75-8 Acrylic acid, polymer with acrylamide and diallyldimethylam 3 25084-90-6 Acrylic acid, t-butyl ester, polymer with ethylene 3 25036-25-3 Acrylonitrile-methyl methacrylate-vinylidene chloride copoly 3 1406-16-2 Activated ergosterol 3 124-04-9 Adipic acid 3 9010-89-3 Adipic acid, polymer with diethylene glycol 3 9002-18-0 Agar 3 61791-56-8 beta- Alanine, N-(2-carboxyethyl)-, N-tallow alkyl derivs., disodium3 14960-06-6 beta- Alanine, N-(2-carboxyethyl)-N-dodecyl-, monosodium salt 3 Alanine, N-coco alkyl derivs. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Old Time Chemical Names
Old Time Chemical Names 1080------------------------------------------------Sodium fluroacetate 2-Propanone-------------------------------------Acetone Absinthe-------------------------------------------Distillate of worm wood Abstinthium--------------------------------------Distillate of worm wood Acarcia gum-------------------------------------Gum arabic Acetaldehyde------------------------------------Acetic aldehyde Acetate of alumina-----------------------------Aluminium acetate Acetate of ammonia---------------------------Ammonium acetate Acetate of amyl---------------------------------Amyl acetate Acetate of baryta-------------------------------Barium acetate Acetate of cobalt-------------------------------Cobalt acetate Acetate of copper------------------------------Copper acetate Acetate of ethyl---------------------------------Ethyl acetate Acetate of iron----------------------------------Iron acetate Acetate of lead----------------------------------Lead acetate Acetate of lime----------------------------------Calcium acetate Acetate of manganese------------------------Manganous acetate Acetate of oxide of ethyl---------------------Ethyl acetate Acetate of potassa-----------------------------Potassium acetate Acetate of potassium--------------------------Potassium acetate Acetate of protoxide of Manganese-------Manganous acetate Acetate of soda---------------------------------Sodium acetate Acetate of zinc----------------------------------Zinc acetate Acetic aid basic bismuth---------------------Bismuth subacetate CH3COOBiO Acetic -

Structural Modification of Trans-Cinnamic Acid Using Colletotrichum Acutatum
Rev. Fac. Ing. Univ. Antioquia N.° 63 pp. 20-29. Junio, 2012 Structural modification of trans-cinnamic acid using Colletotrichum acutatum Modificación estructural de ácidotrans -cinámico empleando Colletotrichum acutatum Rodrigo Velasco B.1, Jesús H. Gil G.1, 2, Carlos M. García P.1, Diego L. Durango R.1,* 1Grupo de Química de los Productos Naturales y los Alimentos. Facultad de Ciencias. Escuela de Química. Universidad Nacional de Colombia. Calle 59ª 63-020 Autopista Norte. AA 3840. Medellín, Colombia. 2Departamento de Ingeniería Agrícola y Alimentos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia. Calle 64 x Carrera 65 Autopista Norte. AA 3840. Medellín, Colombia. (Recibido el 18 de febrero de 2011. Aceptado el 23 de mayo de 2012) Abstract The biotransformation of trans-cinnamic acid by whole cells of the Colombian native phytopathogenic fungus Colletotrichum acutatum was studied. Initially, fungitoxicity of this compound against C. acutatum was evaluated; trans-cinnamic acid exhibited a moderate to weak toxicity against the microorganism and apparently a detoxification mechanism was present. Then, in order to study such mechanism and explore the capacity of this fungus to biotransform trans-cinnamic acid into value-added products, the microorganism was incubated with the substrate using three different culture media (Czapeck-Dox, Sabouraud and PDB) at room conditions. Using Czapeck-Dox medium, whole cultures of C. acutatum reduced trans-cinnamic acid, first to aldehydes (trans-cinnamaldehyde and 3-phenylpropanal), then to alcohols (cinnamyl alcohol and 3-phenyl-1-propanol). Subsequently, these alcohols were transformed to the corresponding acetyl esters. Nevertheless, some of these products were absent or present at different concentration when culture medium was changed.