Locations and Chemistries of Sympathetic Nerve Cells That Project to the Gastrointestinal Tract and Spleen*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Autonomic Nervous System
AUTONOMIC NERVOUS SYSTEM PAGE 1 AUTONOMIC NERVOUS SYSTEM PAGE 2 AUTONOMIC NERVOUS SYSTEM PAGE 3 AUTONOMIC NERVOUS SYSTEM PAGE 4 AUTONOMIC NERVOUS SYSTEM PAGE 5 AUTONOMIC NERVOUS SYSTEM PAGE 6 AUTONOMIC NERVOUS SYSTEM PAGE 7 AUTONOMIC NERVOUS SYSTEM PAGE 8 AUTONOMIC NERVOUS SYSTEM PAGE 9 REVIEW QUESTIONS 1. The autonomic nervous system controls the activity of _?_. (a) smooth muscle (b) cardiac muscle (c) glands (d) all of these (e) none of these 2. All preganglionic and postganglionic autonomic neurons are _?_ neurons. (a) somatic efferent (b) visceral efferent (c) somatic afferent (d) visceral afferent (e) association neurons 3. Which neurotransmitter is released at the synapses between preganglionic and postganglionic autonomic neurons ? (a) epinephrine (b) norepinephrine (c) acetylcholine (d) serotonin (e) oxytocin 4. All preganglionic sympathetic neurons are located in: (a) the lateral horn of the spinal cord of spinal cord segments T1-L2 (b) brainstem nuclei (c) intramural (terminal) ganglia (d) paravertebral ganglia of the sympathetic chains (e) prevertebral ganglia 5. All preganglionic parasympathetic neurons are located in _?_. (a) prevertebral ganglia (b) the lateral horn of spinal cord segments T1-L2 (c) sympathetic chain ganglia (d) intramural ganglia (e) brainstem nuclei and spinal cord segments S2-S4 6. Prevertebral and paravertebral ganglia contain _?_. (a) preganglionic sympathetic neurons (b) preganglionic parasympathetic neurons (c) postganglionic sympathetic neurons (d) postganglionic parasympathetic neurons (e) all of these 7. The otic, ciliary, submandibular and pterygopalatine ganglia are located in the head region and contain _?_. (a) preganglionic sympathetic neurons (b) preganglionic parasympathetic neurons (c) postganglionic sympathetic neurons (d) postganglionic parasympathetic neurons (e) none of these 8. -
Nervous System Central Nervous System Peripheral Nervous System Brain Spinal Cord Sensory Division Motor Division Somatic Nervou
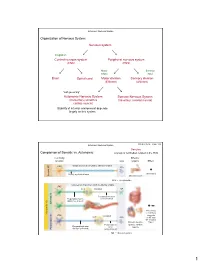
Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) “self governing” Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Stability of internal environment depends largely on this system Marieb & Hoehn – Figure 14.2 Autonomic Nervous System Ganglion: Comparison of Somatic vs. Autonomic: A group of cell bodies located in the PNS Cell body Effector location NTs organs Effect CNS Single neuron from CNS to effector organs ACh + Stimulatory Heavily myelinated axon Somatic NS Somatic Skeletal muscle ACh = Acetylcholine Two-neuron chain from CNS to effector organs CNS ACh Ganglion NE Postganglionic axon Preganglionic axon (unmyelinated) (lightly myelinated) Sympathetic + Stimulatory Autonomic NS Autonomic or inhibitory CNS Ganglion (depends ACh ACh on NT and NT receptor Smooth muscle, Type) Postganglionic glands, cardiac Preganglionic axon axon muscle Parasympathetic (lightly myelinated) (unmyelinated) NE = Norepinephrine 1 Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Sympathetic division -

The Neuroanatomy of Female Pelvic Pain
Chapter 2 The Neuroanatomy of Female Pelvic Pain Frank H. Willard and Mark D. Schuenke Introduction The female pelvis is innervated through primary afferent fi bers that course in nerves related to both the somatic and autonomic nervous systems. The somatic pelvis includes the bony pelvis, its ligaments, and its surrounding skeletal muscle of the urogenital and anal triangles, whereas the visceral pelvis includes the endopelvic fascial lining of the levator ani and the organ systems that it surrounds such as the rectum, reproductive organs, and urinary bladder. Uncovering the origin of pelvic pain patterns created by the convergence of these two separate primary afferent fi ber systems – somatic and visceral – on common neuronal circuitry in the sacral and thoracolumbar spinal cord can be a very dif fi cult process. Diagnosing these blended somatovisceral pelvic pain patterns in the female is further complicated by the strong descending signals from the cerebrum and brainstem to the dorsal horn neurons that can signi fi cantly modulate the perception of pain. These descending systems are themselves signi fi cantly in fl uenced by both the physiological (such as hormonal) and psychological (such as emotional) states of the individual further distorting the intensity, quality, and localization of pain from the pelvis. The interpretation of pelvic pain patterns requires a sound knowledge of the innervation of somatic and visceral pelvic structures coupled with an understand- ing of the interactions occurring in the dorsal horn of the lower spinal cord as well as in the brainstem and forebrain. This review will examine the somatic and vis- ceral innervation of the major structures and organ systems in and around the female pelvis. -

Thoracic Anatomy Autonomic Nervous System
Thoracic anatomy Autonomic nervous system Thoracic Anatomy 3.G.1.1 James Mitchell (December 24, 2003) Autonomic nervous system Division by direction Visceral efferent Preganglionic myelinated, postganglionic unmyelinated Synapse in ganglia Visceral afferent Similar to somatic afferent Cell body in CNS, peripheral processes travel with autonomic and somatic fibres Division by outflow Sympathetic Thoracolumbar outflow: T1-L3 Synapse in sympathetic trunk ganglia or other ganglia near CNS Preganglionic cholinergic, postganglionic predominantly noradrenergic (also adrenergic, cholinergic sudomotor and purinergic) Parasympathetic Craniosacral outflow: III, VII, IX, X, S2-4 Synapse adjacent to end-organs Cranial nerve parasympathetic ganglia are traversed by other fibres but contain only parasympathetic synapses Parasympathetic anatomy III Edinger-Westphal nuclei → oculomotor n. → n. to inferior oblique → ciliary ganglion → short ciliary nn. → ciliary muscle and sphincter pupillae VII Superior salivatory nucleus → nervus intermedius → facial n. → chorda tympani → lingual n. → submandibular ganglion → submandibular and sublingual glands Geniculate ganglion → greater petrosal n. → pterygopalatine ganglion → zygomatic and lacrimal nerves to lacrimal gland and nasal and palatine branches to nasal mucosa XI Inferior salivatory nucleus → glossopharyngeal nerve → tympanic plexus → lesser petrosal n. → otic ganglion → auriculotemporal n. → parotid gland and oral mucosa X Dorsal nucleus of vagus → vagus n. → minute ganglia in respiratory tract, heart, -

Autonomic Nervous System ANS 1 Introduction
Autonomic Nervous System ANS 1 Introduction Control the visceral function = arterial pressure = gastrointestinal motility and secretion = urinary bladder emptying = sweating = body temperature Sympathetic • Divisions of ANS : Division Parasympathetic Division ANS: Preganglionic fibers Postganglionic fibers The Autonomic nervous system Activated by centers located in: • -the spinal cord • - brain stem • - hypothalamus • - the limbic cortex • - The Autonomic nervous system operates by means of visceral reflexes - The efferent Autonomic signals are transmitted to the body through two major subdivisions called - The sympathetic nervous system . - The parasympathetic nervous system. Sympathetic Division Physiological Anatomy of the sympathetic nervous system - two para vertebral sympathetic chains of ganglia in sides of the spinal column - two pre vertebral ganglia inside the abdomen and nervous extending from the ganglia to the different internal organs - the sympathetic nervous originate in the spinal cord between the segments T1 - L2 and pass from here first in to the sympathetic chain and then to the tissues and organs The cell body of each pre ganglion nervous lies in the inter media lateral from of the spinal cord , and its fibers passes through an anterior root of the cord and the spinal nerve. The pre ganglion sympathetic fibers leave the nerve and pass into one of the ganglia of the sympathetic chain Sympathetic Ganglia: A - Paravertebral or Sympathetic chain ganglia B - Prevertebral or Collateral ganglia C - Terminal or Peripheral -

Sympathetic Tales: Subdivisons of the Autonomic Nervous System and the Impact of Developmental Studies Uwe Ernsberger* and Hermann Rohrer
Ernsberger and Rohrer Neural Development (2018) 13:20 https://doi.org/10.1186/s13064-018-0117-6 REVIEW Open Access Sympathetic tales: subdivisons of the autonomic nervous system and the impact of developmental studies Uwe Ernsberger* and Hermann Rohrer Abstract Remarkable progress in a range of biomedical disciplines has promoted the understanding of the cellular components of the autonomic nervous system and their differentiation during development to a critical level. Characterization of the gene expression fingerprints of individual neurons and identification of the key regulators of autonomic neuron differentiation enables us to comprehend the development of different sets of autonomic neurons. Their individual functional properties emerge as a consequence of differential gene expression initiated by the action of specific developmental regulators. In this review, we delineate the anatomical and physiological observations that led to the subdivision into sympathetic and parasympathetic domains and analyze how the recent molecular insights melt into and challenge the classical description of the autonomic nervous system. Keywords: Sympathetic, Parasympathetic, Transcription factor, Preganglionic, Postganglionic, Autonomic nervous system, Sacral, Pelvic ganglion, Heart Background interplay of nervous and hormonal control in particular The “great sympathetic”... “was the principal means of mediated by the sympathetic nervous system and the ad- bringing about the sympathies of the body”. With these renal gland in adapting the internal -

Ganglion of Impar Block
Ganglion of Impar Block A ganglion of impar block is safe and easy procedure used to treat visceral, pelvic, genital, perineal and anal pain. This injection is considered to be a type of sympathetic block that can be used in the treatment of sympathetically-mediated pain, pain secondary to malignancy, neuropathic pain and post- surgical pain. Patients who will benefit from this blockade will frequently present with vague and poorly localized pain in the “seat” region, which is burning in character and frequently accompanied by sensations of urgency with urination and/or defecation.[1] The target in the procedure is the ganglion of impar – also known as the ganglion of Walther or sacrococcygeal ganglion. It is a singular retroperitoneal structure located at the level of the sacrococcygeal junction (SCJ). There are 4 or 5 small sacral ganglia with the ganglion Impar being the most caudal segment of the confluence of the sacral sympathetic chain as it passes anteromedially over the sacrum. More specifically, the ganglion Impar is the terminal fusion of the 2 sacral sympathetic chains and is located with some anatomical variability between the SCJ and the lower segment of the first coccyx. The fusion of the 2 chains typically positions the ganglion midline, which makes it relatively easy to find. However, there is a wide range of variability in the anatomical location with respect to the SCJ.[2] This structure is of particular importance when considering patients who suffer from pain in the pelvic and perineal structures as it provides nociceptive and sympathetic supply to those regions. It receives afferent innervation from: Perineum Distal rectum Anus Distal urethra Distal vagina Vulva Coccyx Scrotum The block is performed by injecting a small amount of anesthetic onto the ganglion of impar, signals of the sympathetic nervous system (SNS) and pain fibers are interrupted from multiple structures simultaneously, leading to dramatic pain relief. -

Morphology of Sympathetic Chain in Saguinus Niger
Anais da Academia Brasileira de Ciências (2013) 85(1): 365-370 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 www.scielo.br/aabc Morphology of sympathetic chain in Saguinus niger MARINA P.E. PINTO1, ÉRIKA BRANCO1, EMERSON T. FIORETTO2, LUIZA C. PEREIRA3 and ANA R. LIMA1 1Universidade Federal Rural da Amazônia (UFRA), Instituto de Saúde e Produção Animal – ISPA, Faculdade de Medicina Veterinária, Avenida Perimetral, 2501, Belém, PA, Brasil 2 Universidade Federal de Sergipe (UFS), Cidade Universitária Professor José Aloísio de Campos, Avenida Marechal Rondon, s/n, Jardim Rosa Elze, São Cristovão, Aracajú, SE, Brasil 3 Empresa Hydro LTDA, Mina de Bauxita – Paragominas, PA, Brasil Manuscript received on March 20, 2012; accepted for publication on October 2, 2012 ABSTRACT Saguinus niger popularly known as Sauim, is a Brazilian North primate. Sympathetic chain investigation would support traumatic and/or cancer diagnosis which are little described in wild animals. The aim of this study was to describe the morphology and distribution of sympathetic chain in order to supply knowledge for neurocomparative research. Three female young animals that came death by natural causes were investigated. Animals were fixed in formaldehyde 10% and dissected along the sympathetic chain in neck, thorax and abdomen. Cranial cervical ganglion was located at the level of carotid bifurcation, related to carotid internal artery. In neck basis the vagosympathetic trunk divides into the sympathetic trunk and the parasympathetic vagal nerve. Sympathetic trunk ran in dorsal position and originated the stellate ganglia, formed by the fusion of caudal cervical and first thoracic ganglia. -

The Intrinsic Cardiac Nervous System and Its Role in Cardiac Pacemaking and Conduction
Journal of Cardiovascular Development and Disease Review The Intrinsic Cardiac Nervous System and Its Role in Cardiac Pacemaking and Conduction Laura Fedele * and Thomas Brand * Developmental Dynamics, National Heart and Lung Institute (NHLI), Imperial College, London W12 0NN, UK * Correspondence: [email protected] (L.F.); [email protected] (T.B.); Tel.: +44-(0)-207-594-6531 (L.F.); +44-(0)-207-594-8744 (T.B.) Received: 17 August 2020; Accepted: 20 November 2020; Published: 24 November 2020 Abstract: The cardiac autonomic nervous system (CANS) plays a key role for the regulation of cardiac activity with its dysregulation being involved in various heart diseases, such as cardiac arrhythmias. The CANS comprises the extrinsic and intrinsic innervation of the heart. The intrinsic cardiac nervous system (ICNS) includes the network of the intracardiac ganglia and interconnecting neurons. The cardiac ganglia contribute to the tight modulation of cardiac electrophysiology, working as a local hub integrating the inputs of the extrinsic innervation and the ICNS. A better understanding of the role of the ICNS for the modulation of the cardiac conduction system will be crucial for targeted therapies of various arrhythmias. We describe the embryonic development, anatomy, and physiology of the ICNS. By correlating the topography of the intracardiac neurons with what is known regarding their biophysical and neurochemical properties, we outline their physiological role in the control of pacemaker activity of the sinoatrial and atrioventricular nodes. We conclude by highlighting cardiac disorders with a putative involvement of the ICNS and outline open questions that need to be addressed in order to better understand the physiology and pathophysiology of the ICNS. -

Nervous System Central Nervous System Peripheral Nervous System Brain Spinal Cord Sensory Division Motor Division Somatic Nervou
Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) “self governing” Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Stability of internal environment depends largely on this system Marieb & Hoehn – Figure 14.2 Autonomic Nervous System Ganglion: Comparison of Somatic vs. Autonomic: A group of cell bodies located in the PNS Cell body Effector location NTs organs Effect CNS Single neuron from CNS to effector organs ACh + Stimulatory Heavily myelinated axon Somatic NS Somatic Skeletal muscle ACh = Acetylcholine Two-neuron chain from CNS to effector organs CNS ACh Ganglion NE Postganglionic axon Preganglionic axon (unmyelinated) (lightly myelinated) Sympathetic + Stimulatory Autonomic NS Autonomic or inhibitory CNS Ganglion (depends ACh ACh on NT and NT receptor Smooth muscle, Type) Postganglionic glands, cardiac Preganglionic axon axon muscle Parasympathetic (lightly myelinated) (unmyelinated) NE = Norepinephrine Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Sympathetic division -

THE ANATOMY of the SYMPATHETHIC TRUNKS in MAN by MARTIN WRETE Histological Department, the University of Uppsala, Sweden
[ 448 ] THE ANATOMY OF THE SYMPATHETHIC TRUNKS IN MAN BY MARTIN WRETE Histological Department, The University of Uppsala, Sweden INTRODUCTION Even a cursory study of the anatomical descriptions of the cervical parts of the sympathetic trunks given in modern text-books or articles discloses that, now as earlier, great confusion exists with respect to terminology. This applies even to monographs and more specialized presentations. The primary cause of this confusion is the very marked variability of the trunks in the neck region, which gives wide scope for arbitrary interpretations of the arrangement; some uncertainty about the terminology and notation of other parts of the trunks also persists. It is true that the terms to be used for the sympathetic nervous system were fixed by the International Anatomical Nomenclature Committee (Nomina Anatomica, Paris, 1955). This does not, however, prevent some of the individual terms being used to denote different anatomical units, and for practical reasons (such as limiting printing costs) comprehensive explanations could not always be given in the annota- tions to the Parisian Nomina Anatomica. As one of the three members of the Sub- Committee responsible for the nomenclature of the peripheral nervous system, I wish to define more exactly my views on the terminology adopted for the sympathetic trunks. I also take this opportunity of revising a few terms I used in certain papers published some twenty years ago. In Nomina Anatomica the term truncus sympathicus is followed by the names of its ganglia, ganglia trunci sympathici, as well as of its connecting rami interganglio- nares. But, also under the heading ganglia trunci sympathici, the term ganglia intermedia is used to denote ganglia on the rami communicantes and certain ganglia on the trunks in the rami interganglionares between the other ganglia-namely the ganglion cervicale superius, ganglion cervicale medium, ganglion cervicothoracicum (s. -
Autonomic Nervous System
NERVOUS SYSTEM OUTLINE 18.1 Comparison of the Somatic and Autonomic Nervous Systems 540 18.2 Overview of the Autonomic Nervous System 542 18 18.3 Parasympathetic Division 545 18.3a Cranial Nerves 545 18.3b Sacral Spinal Nerves 545 18.3c Effects and General Functions of the Parasympathetic Division 545 Autonomic 18.4 Sympathetic Division 547 18.4a Organization and Anatomy of the Sympathetic Division 547 18.4b Sympathetic Pathways 550 Nervous 18.4c Effects and General Functions of the Sympathetic Division 550 18.5 Other Features of the Autonomic Nervous System 552 System 18.5a Autonomic Plexuses 552 18.5b Neurotransmitters and Receptors 553 18.5c Dual Innervation 554 18.5d Autonomic Reflexes 555 18.6 CNS Control of Autonomic Function 556 18.7 Development of the Autonomic Nervous System 557 MODULE 7: NERVOUS SYSTEM mck78097_ch18_539-560.indd 539 2/14/11 3:46 PM 540 Chapter Eighteen Autonomic Nervous System n a twisting downhill slope, an Olympic skier is concentrat- Recall from figure 14.2 (page 417) that the somatic nervous O ing on controlling his body to negotiate the course faster than system and the autonomic nervous system are part of both the anyone else in the world. Compared to the spectators in the viewing central nervous system and the peripheral nervous system. The areas, his pupils are more dilated, and his heart is beating faster SNS operates under our conscious control, as exemplified by vol- and pumping more blood to his skeletal muscles. At the same time, untary activities such as getting out of a chair, picking up a ball, organ system functions not needed in the race are practically shut walking outside, and throwing the ball for the dog to chase.