The Anatomy of Spinal Sympathetic Structures in the Cat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variations of Thoracic Splanchnic Nerves and Its Clinical Implications
Int. J. Morphol., 23(3):247-251, 2005. Variations of Thoracic Splanchnic Nerves and its Clinical Implications Variaciones de los Nervios Esplácnicos Torácicos y sus Implicancias Clínicas *Tony George Jacob; ** Surbhi Wadhwa; ***Shipra Paul & ****Srijit Das JACOB, G. T.; WADHWA, S.; PAUL, S. & DAS, S. Variations of thoracic splanchnic nerves and its clinical implications. Int. J. Morphol., 23(3):247-251, 2005. SUMMARY:The present study reports an anomalous branching pattern of the thoracic sympathetic chain. At the level of T3 ganglion, an anomalous branch i.e accessory sympathetic chain (ASC) descended anteromedial to the main sympathetic chain (MSC). The MSC and the ASC communicated with each other at the level of T9, T10 and T11 ganglion, indicating the absence of classical pattern of greater, lesser and least splanchnic nerves on the right side. However, on the left side, the sympathetic chain displayed normal branching pattern. We opine that the ASC may be representing a higher origin of greater splanchnic nerve at the level of T3 ganglion and the branches from MSC at T9, T10 and T11 ganglion may be the lesser and least splanchnic nerves, which further joined the ASC (i.e presumably the greater splanchnic nerve) to form a common trunk. This common trunk pierced the right crus of diaphragm to reach the right suprarenal plexus after giving few branches to the celiac plexus. Awareness and knowledge of such anatomical variants of thoracic sympathetic chain may be helpful to surgeons in avoiding any incomplete denervation or preventing any inadvertent injury during thoracic sympathectomy. KEY WORDS: Splanchnic nerves; Sympathetic chain; Trunk thoracic; Ganglion. -

Lecture Notes on Human Anatomy. Part One, Fourth Edition. PUB DATE Sep 89 NOTE 79P.; for Related Documents, See SE 051 219-221
DOCUMENT RESUME ED 315 320 SE 051 218 AUTHOR Conrey, Kathleen TITLE Lecture Notes on Human Anatomy. Part One, Fourth Edition. PUB DATE Sep 89 NOTE 79p.; For related documents, see SE 051 219-221. Black and white illustrations will not reproduce clearly. AVAILABLE FROM Aramaki Design and Publications, 12077 Jefferson Blvd., Culver City, CA 90506 ($7.75). PUB TYPE Guides - Classroom Use - Materials (For Learner) (051) EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Anatomy; *Biological Sciences; *College Science; Higher Education; *Human Body; *Lecture Method; Science Education; Secondary Education; Secondary School Science; Teaching Guides; Teaching Methods ABSTRACT During the process of studying the specific course content of human anatomy, students are being educated to expand their vocabulary, deal successfully with complex tasks, anduse a specific way of thinking. This is the first volume in a set of notes which are designed to accompany a lecture series in human anatomy. This volume Includes discussions of anatomical planes and positions, body cavities, and architecture; studies of the skeleton including bones and joints; studies of the musculature of the body; and studiesof the nervous system including the central, autonomic, motor and sensory systems. (CW) *****1.**k07********Y*******t1.****+***********,****A*******r****** % Reproductions supplied by EDRS are the best that can be made from the original document. **************************************************************A**t***** "PERMISSION TO REPRODUCE -

The Nerve Lesion in the Carpal Tunnel Syndrome
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.39.7.615 on 1 July 1976. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1976, 39, 615-626 The nerve lesion in the carpal tunnel syndrome SYDNEY SUNDERLAND From the Department of Experimental Neurology, University of Melbourne, Parkville, Victoria, Australia SYNOPSIS The relative roles of pressure deformation and ischaemia in the production of compres- sion nerve lesions remain a controversial issue. This paper concerns the genesis of the structural changes which follow compression of the median nerve in the carpal tunnel. The initial lesion is an intrafunicular anoxia caused by obstruction to the venous return from the funiculi as the result of increased pressure in the tunnel. This leads to intrafunicular oedema and an increase in intrafunicular pressure which imperil and finally destroy nerve fibres by impairing their blood supply and by compression. The final outcome is the fibrous tissue replacement of the contents of the funiculi. Protected by copyright. In 1862 Waller described the motor, vasomotor, (Gasser, 1935; Allen, 1938; Bentley and Schlapp, and sensory changes which followed the com- 1943; Richards, 1951; Moldaver, 1954; Gelfan pression of nerves in his own arm. His account and Tarlov, 1956). carried no reference to the mechanism In the 1940s Weiss and his associates (Weiss, responsible for blocking conduction in the nerve 1943, 1944; Weiss and Davis, 1943; Weiss and fibres, presumably because he regarded it as Hiscoe, 1948), who were primarily concerned obvious that pressure was the offending agent. with the technical problem of uniting severed Interest in the effects of nerve compression nerves, observed that a divided nerve enclosed was not renewed until the 1920s when cuff or in a tightly fitting arterial sleeve became swollen tourniquet compression was used to produce a proximal and distal to the constriction. -

Autonomic Nervous System
AUTONOMIC NERVOUS SYSTEM PAGE 1 AUTONOMIC NERVOUS SYSTEM PAGE 2 AUTONOMIC NERVOUS SYSTEM PAGE 3 AUTONOMIC NERVOUS SYSTEM PAGE 4 AUTONOMIC NERVOUS SYSTEM PAGE 5 AUTONOMIC NERVOUS SYSTEM PAGE 6 AUTONOMIC NERVOUS SYSTEM PAGE 7 AUTONOMIC NERVOUS SYSTEM PAGE 8 AUTONOMIC NERVOUS SYSTEM PAGE 9 REVIEW QUESTIONS 1. The autonomic nervous system controls the activity of _?_. (a) smooth muscle (b) cardiac muscle (c) glands (d) all of these (e) none of these 2. All preganglionic and postganglionic autonomic neurons are _?_ neurons. (a) somatic efferent (b) visceral efferent (c) somatic afferent (d) visceral afferent (e) association neurons 3. Which neurotransmitter is released at the synapses between preganglionic and postganglionic autonomic neurons ? (a) epinephrine (b) norepinephrine (c) acetylcholine (d) serotonin (e) oxytocin 4. All preganglionic sympathetic neurons are located in: (a) the lateral horn of the spinal cord of spinal cord segments T1-L2 (b) brainstem nuclei (c) intramural (terminal) ganglia (d) paravertebral ganglia of the sympathetic chains (e) prevertebral ganglia 5. All preganglionic parasympathetic neurons are located in _?_. (a) prevertebral ganglia (b) the lateral horn of spinal cord segments T1-L2 (c) sympathetic chain ganglia (d) intramural ganglia (e) brainstem nuclei and spinal cord segments S2-S4 6. Prevertebral and paravertebral ganglia contain _?_. (a) preganglionic sympathetic neurons (b) preganglionic parasympathetic neurons (c) postganglionic sympathetic neurons (d) postganglionic parasympathetic neurons (e) all of these 7. The otic, ciliary, submandibular and pterygopalatine ganglia are located in the head region and contain _?_. (a) preganglionic sympathetic neurons (b) preganglionic parasympathetic neurons (c) postganglionic sympathetic neurons (d) postganglionic parasympathetic neurons (e) none of these 8. -

Of the Pediatric Mediastinum
MRI of the Pediatric Mediastinum Dianna M. E. Bardo, MD Director of Body MR & Co-Director of the 3D Innovation Lab Disclosures Consultant & Speakers Bureau – honoraria Koninklijke Philips Healthcare N V Author – royalties Thieme Publishing Springer Publishing Mediastinum - Anatomy Superior Mediastinum thoracic inlet to thoracic plane thoracic plane to diaphragm Inferior Mediastinum lateral – pleural surface anterior – sternum posterior – vertebral bodies Mediastinum - Anatomy Anterior T4 Mediastinum pericardium to sternum Middle Mediastinum pericardial sac Posterior Mediastinum vertebral bodies to pericardium lateral – pleural surface superior – thoracic inlet inferior - diaphragm Mediastinum – MR Challenges Motion Cardiac ECG – gating/triggering Breathing Respiratory navigation Artifacts Intubation – LMA Surgical / Interventional materials Mediastinum – MR Sequences ECG gated/triggered sequences SSFP – black blood SE – IR – GRE Non- ECG gated/triggered sequences mDIXON (W, F, IP, OP), eTHRIVE, turbo SE, STIR, DWI Respiratory – triggered, radially acquired T2W MultiVane, BLADE, PROPELLER Mediastinum – MR Sequences MRA / MRV REACT – non Gd enhanced Gd enhanced sequences THRIVE, mDIXON, mDIXON XD Mediastinum – Contents Superior Mediastinum PVT Left BATTLE: Phrenic nerve Vagus nerve Structures at the level of the sternal angle Thoracic duct Left recurrent laryngeal nerve (not the right) CLAPTRAP Brachiocephalic veins Cardiac plexus Aortic arch (and its 3 branches) Ligamentum arteriosum Thymus Aortic arch (inner concavity) Trachea Pulmonary -

Deconstructing Spinal Interneurons, One Cell Type at a Time Mariano Ignacio Gabitto
Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy under the Executive Committee of the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 © 2016 Mariano Ignacio Gabitto All rights reserved ABSTRACT Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Abstract Documenting the extent of cellular diversity is a critical step in defining the functional organization of the nervous system. In this context, we sought to develop statistical methods capable of revealing underlying cellular diversity given incomplete data sampling - a common problem in biological systems, where complete descriptions of cellular characteristics are rarely available. We devised a sparse Bayesian framework that infers cell type diversity from partial or incomplete transcription factor expression data. This framework appropriately handles estimation uncertainty, can incorporate multiple cellular characteristics, and can be used to optimize experimental design. We applied this framework to characterize a cardinal inhibitory population in the spinal cord. Animals generate movement by engaging spinal circuits that direct precise sequences of muscle contraction, but the identity and organizational logic of local interneurons that lie at the core of these circuits remain unresolved. By using our Sparse Bayesian approach, we showed that V1 interneurons, a major inhibitory population that controls motor output, fractionate into diverse subsets on the basis of the expression of nineteen transcription factors. Transcriptionally defined subsets exhibit highly structured spatial distributions with mediolateral and dorsoventral positional biases. These distinctions in settling position are largely predictive of patterns of input from sensory and motor neurons, arguing that settling position is a determinant of inhibitory microcircuit organization. -

The Sympathetic and the Parasympathetic Nervous System
The sympathetic and the parasympathetic nervous system Zsuzsanna Tóth, PhD Institute of Anatomy, Histology and Embryology Semmelweis University The role of the autonomic nervous system Claude Bernard • „milieu intérieur” concept; every organism lives in its internal environment that is constant and independent form the external environment Walter Bradford Cannon homeostasis; • an extension of the “milieu interieur” concept • consistence in an open system requires mechanisms that act to maintain that consistency • steady-state conditions require that any tendency toward change automatically meets with factors that resist that change • regulating systems that determine the homeostatic state : o autonomic nervous system ( sympathetic, parasympathetic, enteral) o endocrine system General structure of the autonomic nervous system craniosacral thoracolumbar Anatomy Neurotransmittersof the gut autonomic nervous system. symp. gangl pregangl. fiber pregangl. postgangl. fiber fiber (PoR) PoR enteral ganglion PoR PoR smooth muscle smooth muscle Kuratani S Development 2009;136:1585-1589 Sympathetic activation: Fight or flight reaction • energy mobilization • preparation for escape, or fight vasoconstriction • generalized Parasympathetic activation: adrenal • energy saving and restoring • „rest and digest” system • more localized vasoconstriction Paravertebral ganglia and the sympathetic chains pars cervicalis superius ganglion medium cervicale stellatum pars vertebrae • from the base of the skull to the caudal end thoracalis thoracalis of the sacrum • paravertebral ganglia (ganglia trunci sympathici) • rami interganglionares pars vertebrae • the two chains fuses at the ganglion impar abdominalis lumbalis sacrum pars pelvina foramen sacralia anteriora ganglion impar Anatomy of the cervical part of the sympathetic trunk superior cervical ganglion • behind the seath of the carotid, fusiform ggl. cervicale superius • IML T1-3 vegetative motoneurons- preganglionic fibers truncus symp. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

The Anatomic Basis of Vertebrogenic Pain and the Autonomic Syndrome Associated with Lumbar Disk Extrusion
219 The Anatomic Basis of Vertebrogenic Pain and the Autonomic Syndrome Associated with Lumbar Disk Extrusion 1 2 John R. Jinkins • Extruded lumbar intervertebral disks traditionally have been classified as posterior or Anthony R. Whittemore 1 central in location. A retrospective review of 250 MR imaging examinations of the lumbar William G. Bradley1 spine that used mid- and high-field imagers revealed 145 positive studies, which included a significant number of extrusions extending anteriorly. With the lateral margin of the neural foramen/pedicle as the boundary, 29.2% of peripheral disk extrusions were anterior and 56.4% were posterior. In addition, a prevalence of 14.4% was found for central disk extrusions, in which there was a rupture of disk material into or through the vertebral body itself. The clinical state of neurogenic spinal radiculopathy accom panying posterior disk extrusion has been well defined; however, uncomplicated anterior and central disk extrusions also may be associated with a definite clinical syndrome. The vertebrogenic symptom complex includes (1) local and referred pain and (2) autonomic reflex dysfunction within the lumbosacral zones of Head. Generalized alter ations in viscerosomatic tone potentially may also be observed. The anatomic basis for the mediation of clinical signs and symptoms generated within the disk and paradiskal structures rests with afferent sensory fibers from two primary sources: (1) posterolateral neural branches emanating from the ventral ramus of the somatic spinal root and (2) neural rami projecting directly to the paravertebral autonomic neural plexus. Thus, conscious perception and unconscious effects originating in the vertebral column, although complex, have definite pathways represented in this dual peripheral innervation associated with intimately related andfor parallel central ramifications. -
Nervous System Central Nervous System Peripheral Nervous System Brain Spinal Cord Sensory Division Motor Division Somatic Nervou
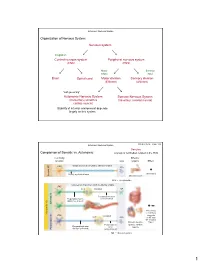
Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) “self governing” Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Stability of internal environment depends largely on this system Marieb & Hoehn – Figure 14.2 Autonomic Nervous System Ganglion: Comparison of Somatic vs. Autonomic: A group of cell bodies located in the PNS Cell body Effector location NTs organs Effect CNS Single neuron from CNS to effector organs ACh + Stimulatory Heavily myelinated axon Somatic NS Somatic Skeletal muscle ACh = Acetylcholine Two-neuron chain from CNS to effector organs CNS ACh Ganglion NE Postganglionic axon Preganglionic axon (unmyelinated) (lightly myelinated) Sympathetic + Stimulatory Autonomic NS Autonomic or inhibitory CNS Ganglion (depends ACh ACh on NT and NT receptor Smooth muscle, Type) Postganglionic glands, cardiac Preganglionic axon axon muscle Parasympathetic (lightly myelinated) (unmyelinated) NE = Norepinephrine 1 Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Sympathetic division -

The Autonomic Nervous System of Selachians. by John Z
The Autonomic Nervous System of Selachians. By John Z. Young, B.A. With 28 Text-figures. CONTENTS. I. INTRODUCTION ......... 571 II. MATERIAL AND METHODS 572 III. ANATOMY AND HISTOLOGY OF THE SYMPATHETIC SYSTEM . 575 1. Nature of the Rami Communicantes .... 575 2. Anterior Eami Communicantes and Sympathetic Ganglia 581 3. Anatomy of the Sympathetic System in the Trunk . 581 4. Sympathetic System and Suprarenals in the Kidney Kegion 586 5. Sympathetic System in the Tail ..... 589 6. Autonomic Fibres in Spinal Dorsal Roots . 593 7. Cytology of the Sympathetic Ganglia .... 593 8. Relation of the Sympathetic Cells to the Suprarenal Tissue 598 9. Post-Branchial Plexus 600 IV. INNERVATION OF THE VISCERA 603 1. Nerves of the Alimentary Canal ..... 603 2. Innervation of the Urinogenital System . 609 3. Cardiac Nerves 610 V. CRANIAL AUTONOMIC SYSTEM 610 1. Autonomic Fibres in Branchial Nerves . 610 2. Profundus and Ciliary Nerves ..... 614 VI. PHYLOGENETIC HISTORY OF THE AUTONOMIC NERVOUS SYSTEM 617 VII. SUMMARY. 621 VIII. BIBLIOGRAPHY 623 I. INTRODUCTION. THE only complete account of the sympathetic nervous system of Selachians is that of Chevrel published in 1887. Since that date several papers have appeared dealing with special points of structure or function, such as those of Bottazzi (1902), Muller and Liljestrand (1918), and Lutz (1981), on the innerva- tion of the viscera; of Diamare (1901) on the histology; and of NO. 300 o o 572 JOHN Z. YOUNG Hoffmann (1900), Miiller (1920), and others, on the development. No attempt has yet been made to investigate the autonomic nervous system of these fish from the general standpoint intro- duced by Langley (1921) and Gaskell (1915); this the present study attempts to do. -

The Neuroanatomy of Female Pelvic Pain
Chapter 2 The Neuroanatomy of Female Pelvic Pain Frank H. Willard and Mark D. Schuenke Introduction The female pelvis is innervated through primary afferent fi bers that course in nerves related to both the somatic and autonomic nervous systems. The somatic pelvis includes the bony pelvis, its ligaments, and its surrounding skeletal muscle of the urogenital and anal triangles, whereas the visceral pelvis includes the endopelvic fascial lining of the levator ani and the organ systems that it surrounds such as the rectum, reproductive organs, and urinary bladder. Uncovering the origin of pelvic pain patterns created by the convergence of these two separate primary afferent fi ber systems – somatic and visceral – on common neuronal circuitry in the sacral and thoracolumbar spinal cord can be a very dif fi cult process. Diagnosing these blended somatovisceral pelvic pain patterns in the female is further complicated by the strong descending signals from the cerebrum and brainstem to the dorsal horn neurons that can signi fi cantly modulate the perception of pain. These descending systems are themselves signi fi cantly in fl uenced by both the physiological (such as hormonal) and psychological (such as emotional) states of the individual further distorting the intensity, quality, and localization of pain from the pelvis. The interpretation of pelvic pain patterns requires a sound knowledge of the innervation of somatic and visceral pelvic structures coupled with an understand- ing of the interactions occurring in the dorsal horn of the lower spinal cord as well as in the brainstem and forebrain. This review will examine the somatic and vis- ceral innervation of the major structures and organ systems in and around the female pelvis.