Herpes Simplex Virusand the Nervous System P. G. E. KENNEDY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Autonomic Nervous System
AUTONOMIC NERVOUS SYSTEM PAGE 1 AUTONOMIC NERVOUS SYSTEM PAGE 2 AUTONOMIC NERVOUS SYSTEM PAGE 3 AUTONOMIC NERVOUS SYSTEM PAGE 4 AUTONOMIC NERVOUS SYSTEM PAGE 5 AUTONOMIC NERVOUS SYSTEM PAGE 6 AUTONOMIC NERVOUS SYSTEM PAGE 7 AUTONOMIC NERVOUS SYSTEM PAGE 8 AUTONOMIC NERVOUS SYSTEM PAGE 9 REVIEW QUESTIONS 1. The autonomic nervous system controls the activity of _?_. (a) smooth muscle (b) cardiac muscle (c) glands (d) all of these (e) none of these 2. All preganglionic and postganglionic autonomic neurons are _?_ neurons. (a) somatic efferent (b) visceral efferent (c) somatic afferent (d) visceral afferent (e) association neurons 3. Which neurotransmitter is released at the synapses between preganglionic and postganglionic autonomic neurons ? (a) epinephrine (b) norepinephrine (c) acetylcholine (d) serotonin (e) oxytocin 4. All preganglionic sympathetic neurons are located in: (a) the lateral horn of the spinal cord of spinal cord segments T1-L2 (b) brainstem nuclei (c) intramural (terminal) ganglia (d) paravertebral ganglia of the sympathetic chains (e) prevertebral ganglia 5. All preganglionic parasympathetic neurons are located in _?_. (a) prevertebral ganglia (b) the lateral horn of spinal cord segments T1-L2 (c) sympathetic chain ganglia (d) intramural ganglia (e) brainstem nuclei and spinal cord segments S2-S4 6. Prevertebral and paravertebral ganglia contain _?_. (a) preganglionic sympathetic neurons (b) preganglionic parasympathetic neurons (c) postganglionic sympathetic neurons (d) postganglionic parasympathetic neurons (e) all of these 7. The otic, ciliary, submandibular and pterygopalatine ganglia are located in the head region and contain _?_. (a) preganglionic sympathetic neurons (b) preganglionic parasympathetic neurons (c) postganglionic sympathetic neurons (d) postganglionic parasympathetic neurons (e) none of these 8. -
Nervous System Central Nervous System Peripheral Nervous System Brain Spinal Cord Sensory Division Motor Division Somatic Nervou
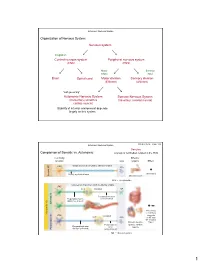
Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) “self governing” Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Stability of internal environment depends largely on this system Marieb & Hoehn – Figure 14.2 Autonomic Nervous System Ganglion: Comparison of Somatic vs. Autonomic: A group of cell bodies located in the PNS Cell body Effector location NTs organs Effect CNS Single neuron from CNS to effector organs ACh + Stimulatory Heavily myelinated axon Somatic NS Somatic Skeletal muscle ACh = Acetylcholine Two-neuron chain from CNS to effector organs CNS ACh Ganglion NE Postganglionic axon Preganglionic axon (unmyelinated) (lightly myelinated) Sympathetic + Stimulatory Autonomic NS Autonomic or inhibitory CNS Ganglion (depends ACh ACh on NT and NT receptor Smooth muscle, Type) Postganglionic glands, cardiac Preganglionic axon axon muscle Parasympathetic (lightly myelinated) (unmyelinated) NE = Norepinephrine 1 Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Sympathetic division -

Autonomic Nervous System ANS 1 Introduction
Autonomic Nervous System ANS 1 Introduction Control the visceral function = arterial pressure = gastrointestinal motility and secretion = urinary bladder emptying = sweating = body temperature Sympathetic • Divisions of ANS : Division Parasympathetic Division ANS: Preganglionic fibers Postganglionic fibers The Autonomic nervous system Activated by centers located in: • -the spinal cord • - brain stem • - hypothalamus • - the limbic cortex • - The Autonomic nervous system operates by means of visceral reflexes - The efferent Autonomic signals are transmitted to the body through two major subdivisions called - The sympathetic nervous system . - The parasympathetic nervous system. Sympathetic Division Physiological Anatomy of the sympathetic nervous system - two para vertebral sympathetic chains of ganglia in sides of the spinal column - two pre vertebral ganglia inside the abdomen and nervous extending from the ganglia to the different internal organs - the sympathetic nervous originate in the spinal cord between the segments T1 - L2 and pass from here first in to the sympathetic chain and then to the tissues and organs The cell body of each pre ganglion nervous lies in the inter media lateral from of the spinal cord , and its fibers passes through an anterior root of the cord and the spinal nerve. The pre ganglion sympathetic fibers leave the nerve and pass into one of the ganglia of the sympathetic chain Sympathetic Ganglia: A - Paravertebral or Sympathetic chain ganglia B - Prevertebral or Collateral ganglia C - Terminal or Peripheral -

Ganglion of Impar Block
Ganglion of Impar Block A ganglion of impar block is safe and easy procedure used to treat visceral, pelvic, genital, perineal and anal pain. This injection is considered to be a type of sympathetic block that can be used in the treatment of sympathetically-mediated pain, pain secondary to malignancy, neuropathic pain and post- surgical pain. Patients who will benefit from this blockade will frequently present with vague and poorly localized pain in the “seat” region, which is burning in character and frequently accompanied by sensations of urgency with urination and/or defecation.[1] The target in the procedure is the ganglion of impar – also known as the ganglion of Walther or sacrococcygeal ganglion. It is a singular retroperitoneal structure located at the level of the sacrococcygeal junction (SCJ). There are 4 or 5 small sacral ganglia with the ganglion Impar being the most caudal segment of the confluence of the sacral sympathetic chain as it passes anteromedially over the sacrum. More specifically, the ganglion Impar is the terminal fusion of the 2 sacral sympathetic chains and is located with some anatomical variability between the SCJ and the lower segment of the first coccyx. The fusion of the 2 chains typically positions the ganglion midline, which makes it relatively easy to find. However, there is a wide range of variability in the anatomical location with respect to the SCJ.[2] This structure is of particular importance when considering patients who suffer from pain in the pelvic and perineal structures as it provides nociceptive and sympathetic supply to those regions. It receives afferent innervation from: Perineum Distal rectum Anus Distal urethra Distal vagina Vulva Coccyx Scrotum The block is performed by injecting a small amount of anesthetic onto the ganglion of impar, signals of the sympathetic nervous system (SNS) and pain fibers are interrupted from multiple structures simultaneously, leading to dramatic pain relief. -

Nervous System Central Nervous System Peripheral Nervous System Brain Spinal Cord Sensory Division Motor Division Somatic Nervou
Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) “self governing” Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Stability of internal environment depends largely on this system Marieb & Hoehn – Figure 14.2 Autonomic Nervous System Ganglion: Comparison of Somatic vs. Autonomic: A group of cell bodies located in the PNS Cell body Effector location NTs organs Effect CNS Single neuron from CNS to effector organs ACh + Stimulatory Heavily myelinated axon Somatic NS Somatic Skeletal muscle ACh = Acetylcholine Two-neuron chain from CNS to effector organs CNS ACh Ganglion NE Postganglionic axon Preganglionic axon (unmyelinated) (lightly myelinated) Sympathetic + Stimulatory Autonomic NS Autonomic or inhibitory CNS Ganglion (depends ACh ACh on NT and NT receptor Smooth muscle, Type) Postganglionic glands, cardiac Preganglionic axon axon muscle Parasympathetic (lightly myelinated) (unmyelinated) NE = Norepinephrine Autonomic Nervous System Organization of Nervous System: Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (Efferent) (Afferent) Autonomic Nervous System Somatic Nervous System (Involuntary; smooth & (Voluntary; skeletal muscle) cardiac muscle) Sympathetic division -

THE ANATOMY of the SYMPATHETHIC TRUNKS in MAN by MARTIN WRETE Histological Department, the University of Uppsala, Sweden
[ 448 ] THE ANATOMY OF THE SYMPATHETHIC TRUNKS IN MAN BY MARTIN WRETE Histological Department, The University of Uppsala, Sweden INTRODUCTION Even a cursory study of the anatomical descriptions of the cervical parts of the sympathetic trunks given in modern text-books or articles discloses that, now as earlier, great confusion exists with respect to terminology. This applies even to monographs and more specialized presentations. The primary cause of this confusion is the very marked variability of the trunks in the neck region, which gives wide scope for arbitrary interpretations of the arrangement; some uncertainty about the terminology and notation of other parts of the trunks also persists. It is true that the terms to be used for the sympathetic nervous system were fixed by the International Anatomical Nomenclature Committee (Nomina Anatomica, Paris, 1955). This does not, however, prevent some of the individual terms being used to denote different anatomical units, and for practical reasons (such as limiting printing costs) comprehensive explanations could not always be given in the annota- tions to the Parisian Nomina Anatomica. As one of the three members of the Sub- Committee responsible for the nomenclature of the peripheral nervous system, I wish to define more exactly my views on the terminology adopted for the sympathetic trunks. I also take this opportunity of revising a few terms I used in certain papers published some twenty years ago. In Nomina Anatomica the term truncus sympathicus is followed by the names of its ganglia, ganglia trunci sympathici, as well as of its connecting rami interganglio- nares. But, also under the heading ganglia trunci sympathici, the term ganglia intermedia is used to denote ganglia on the rami communicantes and certain ganglia on the trunks in the rami interganglionares between the other ganglia-namely the ganglion cervicale superius, ganglion cervicale medium, ganglion cervicothoracicum (s. -

Introduction to the Peripheral Nervous System 2
Introduction to the 2 Peripheral Nervous System toward the neuron’s cell body, and an axon is the long process CONTENTS that carries the action potential away from the cell body. INTRODUCTION Some neurons appear to have only a single process extending PERIPHERAL NERVOUS SYSTEM from only one pole (a differentiated region of the cell body) SPINAL CORD (CENTRAL NERVOUS SYSTEM) that divides into two parts (Fig. 2-1). This type of neuron is OVERVIEW OF THE AUTONOMIC NERVOUS SYSTEM called a pseudounipolar neuron because embryonically it Sympathetic Nervous System develops from a bipolar neuroblast in which the two axons Parasympathetic Nervous System fuse. Multipolar neurons (Fig. 2-2) have multiple dendrites Visceral Afferent Neurons and typically a single axon arising from an enlarged portion REFERRED PAIN of the cell body called the axon hillock. These processes CLASSIFICATION OF NEURONAL FIBERS extend from different poles of the cell body. One neuron communicates with other neurons or glands DEVELOPMENT OF THE SPINAL CORD AND or muscle cells across a junction between cells called a PERIPHERAL NERVOUS SYSTEM synapse. Typically, communication is transmitted across a synapse by means of specific neurotransmitters, such as acetylcholine, epinephrine, and norepinephrine, but in some cases in the CNS by means of electric current passing ●●● INTRODUCTION from cell to cell. Many axons are ensheathed with a substance called The nervous system comprises the central nervous system myelin, which acts as an insulator. Myelinated axons transmit (CNS) and the peripheral nervous system (PNS). The CNS is impulses much faster than nonmyelinated axons. Myelin surrounded and protected by the skull (neurocranium) and consists of concentric layers of lipid-rich material formed vertebral column and consists of the brain and the spinal by the plasma membrane of a myelinating cell. -

Hadeel Abdullah Nadeen AL-Falooji Dena Kofahi Mohammad Hesham
Hadeel Abdullah Nadeen AL-Falooji Dena Kofahi Mohammad Hesham 0 | P a g e Nerves ON THE POSTERIOR ABDOMINAL WALL The Lumbar Plexus The lumbar plexus, which is one of the main nervous pathways supplying the lower limb, is formed in the psoas major muscle -in the abdomen- from the anterior rami of the upper four lumbar spinal nerves (L1-L4). Its branches emerge from the lateral₁ and medial₂ borders of the muscle and from its anterior₃ surface. Branches of the Lumbar Plexus - The iLiohypogastric nerve₁, iLioinguinal nerve₂, Lateral cutaneous nerve of the thigh₃, and femoraL nerve₄ emerge from the Lateral border of the psoas, in that order from above downward. - The obturator nerve₁ and lumbosacral trunk₂ emerge from the medial border of the psoas major. - The genitofemoral₁ nerve emerges from the anterior surface of the psoas major. The lumbosacral trunk: It is formed from the L4 (from the lumbar plexus) and L5 (from the sacral plexus) nerve roots (i.e. the fourth lumbar nerve gives off branches to the sacral plexus forming the lumbosacral trunk). - The ventral (anterior) rami of L1 form the iliohypogastric₁ and ilioinguinal₂ nerves which run between the transversus abdominis muscle and abdominal internal oblique muscle. Then: 1. The iliohypogastric nerve (L1) gives off several motor branches to abdominal muscles and a sensory branch to the skin of the lower part of the anterior abdominal wall above the pubic symphysis. 2. The ilioinguinal nerve (L1) pierces the posterior wall of the inguinal canal and runs along with the spermatic cord (through the canal) to supply the skin of the groin and the scrotum or labium majus. -

The Anatomy of Spinal Sympathetic Structures in the Cat
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 1978 The Anatomy of Spinal Sympathetic Structures in the Cat Kyungsoon Chung Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Anatomy Commons Recommended Citation Chung, Kyungsoon, "The Anatomy of Spinal Sympathetic Structures in the Cat" (1978). Dissertations. 1725. https://ecommons.luc.edu/luc_diss/1725 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1978 Kyungsoon Chung ; THE ANATOMY OF SPINAL SYMPATHETIC STRUCTURES IN THE CAT by Kyungsoon Chung A Dissertation Submitted to the Faculty of the Graduate School of Loyola University of Chicago in Partial Fulfillemnt of the Requirements for the Degree of Doctor of Philosophy April 1978 Dedicated to my parents, Mom and Dad ii ACKNOWLEDGEMENTS I would like to express my sincere appreciation to my adviser, Dr. Faith LaVelle, who gave unsparingly of her time and energy to assist in the fruition of this study. The guidance and comments of Dr. Robert Wurster throughout this study were specially appreciated. I would like to thank the faculty of the Depart- ment of Anatomy for giving me a chance for graduate study and for molding me as a scientist. This dissertation would not have been possible without the help and understanding of my husband and colleague, Jin Mo. -

68Ga-PSMA-HBED-CC Uptake in Cervical, Coeliac and Sacral Ganglia
Journal of Nuclear Medicine, published on January 25, 2018 as doi:10.2967/jnumed.117.204677 1 68Ga‐PSMA‐HBED‐CC uptake in cervical, coeliac and sacral ganglia as an important 2 pitfall in prostate cancer PET imaging 3 4 Christoph Rischpler1*, Teresa I. Beck2*, Shozo Okamoto1,3, Anna M. Schlitter4, Karina Knorr1, Markus 5 Schwaiger1, Jürgen Gschwend5, Tobias Maurer5, Philipp T. Meyer2, Matthias Eiber1 6 7 1Department of Nuclear Medicine, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany 8 2Department of Nuclear Medicine, Medical Center ‐ University of Freiburg, Germany 9 3Department of Nuclear Medicine, Hokkaido University Graduate School of 10 Medicine, Japan 11 4Institute of Pathology, Technical University Munich, Munich, Germany 12 5Department of Urology, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany 13 14 *Both first authors contributed equally to this article. 15 16 Corresponding author: 17 Christoph Rischpler, MD 18 Email: [email protected] 19 Phone: +49 (0) 89 /4140‐6085 20 Klinikum Rechts der Isar, 21 Nuklearmedizinische Klinik und Poliklinik 22 Ismaninger Str. 22 23 81675 München 24 25 Word count (abstract): 348 26 Word count (whole manuscript): 3644 27 28 Short running title: PSMA‐ligand uptake in ganglia 1 29 ABSTRACT 30 31 The study aims to investigate the presence of physiological prostate‐specific membrane 32 antigen (PSMA)‐ligand uptake on positron‐emission‐tomography (PET) in cervical, coeliac and 33 sacral ganglia of the sympathetic trunk as a pitfall for lymph node metastases in prostate 34 cancer imaging. Methods: 407 patients who underwent “Glu‐NH‐CO‐NH‐Lys” radio‐labeled 35 with [68Ga]gallium N,N‐bis[2‐hydroxy‐5‐(carboxyethyl)benzyl]ethylenediamine‐N,N‐diacetic 36 acid (68Ga‐PSMA‐HBED‐CC) PET (combined with a diagnostic computed tomography (CT)) 37 were retrospectively analyzed. -

Autonomic Nervous System of the Pelvis — General Overview
FOLIA MEDICA CRACOVIENSIA Vol. LVIII, 2, 2018: 21–44 PL ISSN 0015-5616 DOI: 10.24425/fmc.2018.124656 Autonomic nervous system of the pelvis — general overview Justyna Sienkiewicz-Zawilińska1, Jarosław Zawiliński1, Lourdes Niroya Kaythampillai1, Marcin Jakiel1, Jacenty Urbaniak1, Tomasz Bereza1, Wojciech Kowalski2, Marios Loukas3, Jerzy Walocha1 1Department of Anatomy, Jagiellonian University Medical College, Kraków, Poland 2Gabinety Lekarskie Wojciech Kowalski, Kraków, Poland 3Department of Anatomy, St. George’s University, Grenada Corresponding author: Jerzy A. Walocha, MD, PhD Department of Anatomy, Jagiellonian University Medical College ul. Kopernika 12, 31-034 Kraków, Poland Phone: +48 12 422 95 11; E-mail: [email protected] Abstract: Autonomic nervous system of the pelvis is still poorly understood. Every year more and more pelvic procedures are carried out on patients suff ering from diff erent pelvic disorders what leads to numerous pelvic dysfunctions. Authors tried to review, starting from historical and clinical background, the most important reports on anatomy of the pelvic autonomic plexuses. We also pay attention to complete lack of knowledge of students of medicine on the autonomic nervous structures in the area studied. We present anatomical description of the pelvic plexuses including their visceral branches and anatomy of surrounding pelvic tissues which still remains unclear. More and more attention is paid to the topography of the plexuses specially because of new pain releasing techniques — neurolysies. Key words: autonomic nervous system, inferior hypogastric plexus, anatomy, nerve sparing, pelvis. 22 Justyna Sienkiewicz-Zawilińska, Jarosław Zawiliński, et al. Introduction Th e innervation of human pelvis was a subject of numerous studies and reports of the scientists who studied both fresh and embalmed cadavers (Hunter, Lee, Beck, Frankenhäuser, Davis, Labate, Krantz, Quinn). -
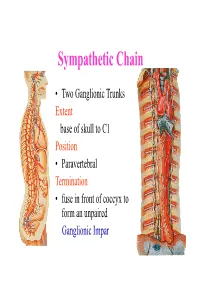
Sympathetic Chain – Cervical Part
Sympathetic Chain • Two Ganglionic Trunks Extent base of skull to C1 Position • Paravertebral Termination • fuse in front of coccyx to form an unpaired Ganglionic Impar Sympathetic Chain •3 Ganglia in cervical part •11Ganglia in thoracic Part •4 lumbar Ganglia •4 Sacral ganglia Sympathetic Chain-cervical part • lie Behind Carotid Sheath and • in front of Longus colli & Longus Capitis muscles • Initially no. of Sympathetic ganglia correspond to no. of Spinal Nerves • Later • Superior formed by fusion of upper 4 cervical Ganglia • Middle by 5th and 6th •Inferiorby joining of 7th and 8th cervical ganglia Sympathetic Chain – Cervical Part • Cervical Part Ganglia Superior Cervical ganglia Middle Cervical Ganglia Inferior Cervical ganglia Sometimes Inferior cervical and first Thoracic fuse to form a Cervico-Thoracic or Stellate Ganglia Sympathetic Chain – Cervical Part • Do not receive white rami communicantes from cervical spinal segments • LAT. HORN CELLS OF T1-T5 PROVIDE PRE- GANGLIONIC FIBRES • Gives grey rami communicantes to all 8 cervical nerves Sympathetic Chain – Cervical Part • GANGLION • Contains-multipolar post ganglionic neurons & few interneurons (chromaffin or SIF cells*) • *modulate activities of post ganglionic neurons by dopamine • SYMPATHETIC TRUNK conveys pre & post ganglionic motor & sensory fibres between ganglia SUPERIOR CERVICAL GANGLION • Largest ,fusiform, 2.5cm length • Fuses upper four cervical ganglia • Situation- opposite C2 &C3 Vertebrae behind ICA& infont of l. capitis • Receives pre ganglionic fibres mostly from upper three thracic segments • BRANCHES (all convey post ganglionic fibres & some sensory fibres) SUPERIOR CERVICAL GANGLION • BRANCHES • Lateral-grey rami comm. to C1- C4 nerves &(C5-C8) • Medial-laryngo-pharyngeal - cardiac(no pain fibr.) Anterior-ramify around CCA,ECA & its branches Ascending-INTERNAL CAROTID NERVE -carotido-tympanic -deep petrosal -communicating(v,iii,iv,v,&vi) -nervus conarii (pineal gland) Term.communicating(ant.