Ecological Studies of the Smooth Earth Snake and Redbelly Snake, and Niche Modeling of Forest Species in Eastern Kansas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Distributions, Vulnerability of Amphibians, T\]Ova Scotia](https://docslib.b-cdn.net/cover/8070/distributions-vulnerability-of-amphibians-t-ova-scotia-18070.webp)
Distributions, Vulnerability of Amphibians, T\]Ova Scotia
Curatorial Report Number 45 Nova Scotia Museum 1747 Summer St. Distributions, Halifax. Nova Scotia. Canada Habitats and Vulnerability of Amphibians, Reptiles,and Small Native Mammals in t\]ova Scotia By John Gil hen and Fred Scott January 1981 Curatorial Report Number 45 Nova Scotia Museum 1747 Summer St. Distributions, Halifax, Nova Scotia, Canada Habitats and Vulnerability of Amphibians, Reptiles, and Small Native Mammals in Nova Scotia By John Gil hen and Fred Scott January 1981 T ~ r T 1 NOVA SCOTIA MUSEUM l Curatorial Reports The Curato~ial Reports of the Nova Scotia Museum contain information , on the collections and the preliminary results of research projects carried out under the program of the museum. The reports may be cited in publications but their manuscript status should be clearly indicated. , 1, 1 l 11 INTRODUCTION In recent years the Science Section of the Nova Scotia Museum has r ecei ved increasingly frequent requests from government and private agencies , and from individuals, for information on plant and animal species or communiti es which are of exceptional scientific value or interest and which may be affected by various kinds of proposed development. These requests fall i nto two categories : information on species or communities occurring at a particular location, and information on the distribution and vulnerability of a species or group of species throughout the province as a whole. Requests in the first category generally cannot be anticipated, and the Science Section r esponds to each one, using a Locality File listing its biological collections by county and location, supplemented by special knowledge of the staff when possible. -
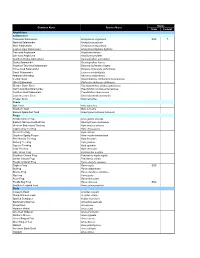
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

What Typical Population Density Could You Expect for the Species in a Hectare of Ideal Habitat?
SQUAMATES DENSITY - What typical population density could you expect for the species in a hectare of ideal habitat? Species Common Name Density Sauria Lizards Anguidae Anguid Lizards Ophisaurus attenuatus longicaudus Eastern Slender Glass Lizard >400 / ha; 4-111/ ha (Fitch 1989) Ophisaurus ventralis Eastern Glass Lizard Unk Lacertidae Wall Lizards Podarcis sicula Italian Wall Lizard Unk Phrynosomatidae Sceloporine Lizards Sceloporus undulatus hyacinthinus Northern Fence Lizard Unk Scincidae Skinks Eumeces a. anthracinus Northern Coal Skink Unk Eumeces fasciatus Common Five-lined Skink 85 / ha (Klemens 1993) Eumeces inexpectatus Southeastern Five-lined Skink Unk Eumeces laticeps Broad-headed Skink Unk Scincella lateralis Ground Skink 400-1500 / ha (Brooks 1967) Teiidae Whiptails Cnemidophorus s. sexlineatus Eastern Six-lined Racerunner 2.5 / 100 m2 (Mitchell 1994) Colubridae Colubrids Carphophis a. amoenus Eastern Worm Snake 60 - 120 / ha in KS (Clark 1970) Cemophora coccinea copei Northern Scarlet Snake Unk Clonophis kirtlandii Kirtland's Snake 19 along 0.6 km street (Minton 1972) Coluber c. constrictor Northern Black Racer 1-3 / ha (Ernst, pers. obs.); 3-7 / ha (Fitch 1963b) Diadophis p. punctatus Southern Ringneck Snake 719-1,849 / ha (Fitch 1975); > 100 / ha (Hulse, pers. obs.) Diadophis p. edwardsii Northern Ringneck Snake 719-1,849 / ha (Fitch 1975); > 100 / ha (Hulse, pers. obs.) Elaphe guttata Corn Snake less than 1 / 100 ha in KS (Fitch 1958a) Elaphe o. obsoleta Black Rat Snake 0.23 / ha MD (Stickel et al. 1980); 1 / ha in KS (Fitch 1963a) Farancia a. abacura Eastern Mud Snake about 150 / km (Hellman and Telford 1956) Farancia e. erytrogramma Common Rainbow Snake 8 in 30 m (Mount 1975); 20 in 4.1 ha (Richmond 1945) Heterodon platirhinos Eastern Hog-nosed Snake 2.1 / ha (Platt 1969); 4.8 / ha in VA (Scott 1986) Lampropeltis calligaster Mole Kingsnake 1 / 2.6 ha (Ernst and Barbour 1989) rhombomaculata Lampropeltis g. -

Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Gulsby Kennesaw State University
Kennesaw State University DigitalCommons@Kennesaw State University Department of Ecology, Evolution, and Organismal Master of Science in Integrative Biology Theses Biology Summer 7-25-2019 Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Gulsby Kennesaw State University Follow this and additional works at: https://digitalcommons.kennesaw.edu/integrbiol_etd Part of the Integrative Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Gulsby, Miranda, "Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia" (2019). Master of Science in Integrative Biology Theses. 48. https://digitalcommons.kennesaw.edu/integrbiol_etd/48 This Thesis is brought to you for free and open access by the Department of Ecology, Evolution, and Organismal Biology at DigitalCommons@Kennesaw State University. It has been accepted for inclusion in Master of Science in Integrative Biology Theses by an authorized administrator of DigitalCommons@Kennesaw State University. For more information, please contact [email protected]. Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Louise Gulsby A Thesis Presented in Partial Fulfillment of Requirements of the Master of Science in Integrative Biology for the Department of Evolution, Ecology, and Organismal Biology Kennesaw State University 1000 Chastain Road Kennesaw, Ga 30144 July 2019 Major Advisor: Thomas McElroy, Ph. D. Committee Members: Joel McNeal, Ph. -

Significant New Records of Amphibians and Reptiles from Georgia, USA
GEOGRAPHIC DISTRIBUTION 597 Herpetological Review, 2015, 46(4), 597–601. © 2015 by Society for the Study of Amphibians and Reptiles Significant New Records of Amphibians and Reptiles from Georgia, USA Distributional maps found in Amphibians and Reptiles of records for a variety of amphibian and reptile species in Georgia. Georgia (Jensen et al. 2008), along with subsequent geographical All records below were verified by David Bechler (VSU), Nikole distribution notes published in Herpetological Review, serve Castleberry (GMNH), David Laurencio (AUM), Lance McBrayer as essential references for county-level occurrence data for (GSU), and David Steen (SRSU), and datum used was WGS84. herpetofauna in Georgia. Collectively, these resources aid Standard English names follow Crother (2012). biologists by helping to identify distributional gaps for which to target survey efforts. Herein we report newly documented county CAUDATA — SALAMANDERS DIRK J. STEVENSON AMBYSTOMA OPACUM (Marbled Salamander). CALHOUN CO.: CHRISTOPHER L. JENKINS 7.8 km W Leary (31.488749°N, 84.595917°W). 18 October 2014. D. KEVIN M. STOHLGREN Stevenson. GMNH 50875. LOWNDES CO.: Langdale Park, Valdosta The Orianne Society, 100 Phoenix Road, Athens, (30.878524°N, 83.317114°W). 3 April 1998. J. Evans. VSU C0015. Georgia 30605, USA First Georgia record for the Suwannee River drainage. MURRAY JOHN B. JENSEN* CO.: Conasauga Natural Area (34.845116°N, 84.848180°W). 12 Georgia Department of Natural Resources, 116 Rum November 2013. N. Klaus and C. Muise. GMNH 50548. Creek Drive, Forsyth, Georgia 31029, USA DAVID L. BECHLER Department of Biology, Valdosta State University, Valdosta, AMBYSTOMA TALPOIDEUM (Mole Salamander). BERRIEN CO.: Georgia 31602, USA St. -

Reptiles in Arkansas
Terrestrial Reptile Report Carphophis amoenus Co mmon Wor msnake Class: Reptilia Order: Serpentes Family: Colubridae Priority Score: 19 out of 100 Population Trend: Unknown Global Rank: G5 — Secure State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Arkansas Valley Ouachita Mountains South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plain Carphophis amoenus Common Wormsnake 1079 Terrestrial Reptile Report Habitat Map Habitats Weight Crowley's Ridge Loess Slope Forest Obligate Lower Mississippi Flatwoods Woodland and Forest Suitable Problems Faced KNOWN PROBLEM: Habitat loss due to conversion Threat: Habitat destruction or to agriculture. conversion Source: Agricultural practices KNOWN PROBLEM: Habitat loss due to forestry Threat: Habitat destruction or practices. conversion Source: Forestry activities Data Gaps/Research Needs Genetic analyses comparing Arkansas populations with populations east of the Mississippi River and the Western worm snake. Conservation Actions Importance Category More data are needed to determine conservation actions. Monitoring Strategies More information is needed to develop a monitoring strategy. Carphophis amoenus Common Wormsnake 1080 Terrestrial Reptile Report Comments Trauth and others (2004) summarized the literature and biology of this snake. In April 2005, two new geographic distribution records were collected in Loess Slope Forest habitat within St. Francis National Forest, south of the Mariana -

Biodiversity from Caves and Other Subterranean Habitats of Georgia, USA
Kirk S. Zigler, Matthew L. Niemiller, Charles D.R. Stephen, Breanne N. Ayala, Marc A. Milne, Nicholas S. Gladstone, Annette S. Engel, John B. Jensen, Carlos D. Camp, James C. Ozier, and Alan Cressler. Biodiversity from caves and other subterranean habitats of Georgia, USA. Journal of Cave and Karst Studies, v. 82, no. 2, p. 125-167. DOI:10.4311/2019LSC0125 BIODIVERSITY FROM CAVES AND OTHER SUBTERRANEAN HABITATS OF GEORGIA, USA Kirk S. Zigler1C, Matthew L. Niemiller2, Charles D.R. Stephen3, Breanne N. Ayala1, Marc A. Milne4, Nicholas S. Gladstone5, Annette S. Engel6, John B. Jensen7, Carlos D. Camp8, James C. Ozier9, and Alan Cressler10 Abstract We provide an annotated checklist of species recorded from caves and other subterranean habitats in the state of Georgia, USA. We report 281 species (228 invertebrates and 53 vertebrates), including 51 troglobionts (cave-obligate species), from more than 150 sites (caves, springs, and wells). Endemism is high; of the troglobionts, 17 (33 % of those known from the state) are endemic to Georgia and seven (14 %) are known from a single cave. We identified three biogeographic clusters of troglobionts. Two clusters are located in the northwestern part of the state, west of Lookout Mountain in Lookout Valley and east of Lookout Mountain in the Valley and Ridge. In addition, there is a group of tro- globionts found only in the southwestern corner of the state and associated with the Upper Floridan Aquifer. At least two dozen potentially undescribed species have been collected from caves; clarifying the taxonomic status of these organisms would improve our understanding of cave biodiversity in the state. -

Muller Hill Unit Management Plan- Final
New York State Department of Environmental Conservation Division of Lands & Forests MULLER HILL UNIT MANAGEMENT PLAN FINAL Towns of Georgetown, DeRuyter, Lincklaen, Otselic, and Cuyler, in Madison, Chenango and Cortland Counties MARCH 2012 NYS Department of Environmental Conservation Region 7 Sub Office 2715 State Highway 80 Sherburne, NY 13460 (607) 674- 4036 ANDREW CUOMO , Governor JOSEPH MARTENS , Commissioner ROBERT DAVIES, State Forester ANDREW M. CUOMO JOE MARTENS GOVERNOR COMMISSIONER STATE OF NEW YORK DEPARTMENT OF ENVIRONMENTAL CONSERVATION ALBANY, NEW YORK 12233-1010 MEMORANDUM TO: The Record FROM: Joseph J. MartensD~ DATE: MAR - 9 2012 SUBJECT: Final Muller Hill UMP The Unit Management Plan for Muller Hill UMP has been completed. The Plan is consistent with Department policy and procedure, involved public participation and is consistent with the Environmental Conservation Law, Rules and Regulations. The plan includes management objectives for a ten year period and is hereby approved and adopted. FINAL Muller Hill Unit Management Plan A Management Unit Consisting of Three State Forests in Southwestern Madison and Northwestern Chenango Counties Prepared By: Greg Owens, Senior Forester Jason Schoellig, Senior Forester New York State Department of Environmental Conservation Lands & Forests Office 2715 State Highway 80 Sherburne, New York 13460 607-674-4036 P r e f a c e It is the policy of the Department to manage State Forests for multiple uses to serve the People of New York State. The Muller Hill Unit Management Plan is the basis for supporting a multiple use* goal through the implementation of specific objectives and management strategies. This management will be carried out to ensure the sustainability, biological improvement and protection of the Unit’s ecosystems and to optimize the many benefits to the public that these State Forests provide. -

ACTION: Original DATE: 01/29/2010 1:24 PM
ACTION: Original DATE: 01/29/2010 1:24 PM TO BE RESCINDED 1501:31-25-04 Reptiles and amphibians regulations. (A) "Native" reptiles or amphibians are those taxa listed in Ohio Revised Code section 1531.01 (WW) and (XX) and includes any individual, parts, eggs, tadpoles, or offspring dead or alive. (B) No endangered reptile or amphibian designated as such may be possessed, bred, sold, offered for sale, traded or bartered except in accordance with Ohio Revised Code section 1531.25 and Ohio Administrative Code rules 1501:31-23-01 and 1501:31-25-04 (this rule). (C) Except as provided in paragraph (B) of this rule, an Ohio resident, with a propagating license, may possess native reptiles or amphibians, live or dead which have been wild captured, legally obtained from out of state or captively produced. The following are exceptions to this rule: (1) The following taxa shall be taken, bought and/or sold in accordance with Ohio Administrative Code rule 1501:31-13-05: Bullfrog (Rana catesbeiana) Green frog (Rana clamitans) Snapping turtle (Chelydra serpentina serpentina) Spiny softshell turtle (Apalone spinifera spinifera) Smooth softshell turtle (Apalone mutica mutica) (2) Except as provided in paragraph (B) of this rule and paragraph (B) of rule 1501:31-13-05 of the Administrative Code, an Ohio resident 17 years of age or younger may possess without a propagating license not more than four individuals or 25 eggs, tadpoles, or larvae of each native reptile or amphibian, live or dead which have been taken from the wild, legally obtained from out of state or captively produced. -

Okefenokee National Wildlife Refuge Amphibians, Fish, Mammals and Reptiles List
U.S. Fish & Wildlife Service Okefenokee National Wildlife Refuge Amphibians, Fish, Mammals and Reptiles List The Okefenokee swamp is covered with Mammals ___Seminole Bat cypress, blackgum, and bay forests (Lasiurus seminolus). A common bat of scattered throughout a flooded prairie ___Virginia Opossum the Okefenokee which is found hanging in made of grasses, sedges, and various (Didelphis virginiana pigna). Common on Spanish Moss during the day. the swamp edge and the islands within aquatic plants. The peripheral upland and ___Hoary Bat the almost 70 islands within the swamp the Swamp. A night prowler. “Pogo” is often seen by campers. (Lasiurus cinereus cinereus). This are forested with pine interspersed with yellowish-brown bat flies high in the air hardwood hammocks. Lakes of varying ___Southern Short-Tailed Shrew late at night and will hang in trees when sizes and depths, and floating sections (Barina carolinensis). A specimen was resting. It is the largest bat in the East of the peat bed, are also part of the found on Floyds Island June 12, 1921. It and eats mostly moths. Okefenokee terrain. kills its prey with poisonous saliva. ___Northern Yellow Bat People have left their mark on the swamp. ___Least Shrew (Lasiurus intermedius floridanus). A 12-mile long canal was dug into the (Cryptotus parva parva). Rarely seen but Apparently a rare species in the area. It eastern prairies in the 1890’s in a failed probably fairly common. Specimens have likes to feed in groups. attempt to drain the swamp. During the been found on several of the islands, on early 1900’s large amounts of timber were the swamp edge, and in the pine woods ___Evening Bat removed, so that very few areas of virgin around the swamp. -

AG-472-02 Snakes
Snakes Contents Intro ........................................................................................................................................................................................................................1 What are Snakes? ...............................................................1 Biology of Snakes ...............................................................1 Why are Snakes Important? ............................................1 People and Snakes ............................................................3 Where are Snakes? ............................................................1 Managing Snakes ...............................................................3 Family Colubridae ...............................................................................................................................................................................................5 Eastern Worm Snake—Harmless .................................5 Red-Bellied Water Snake—Harmless ....................... 11 Scarlet Snake—Harmless ................................................5 Banded Water Snake—Harmless ............................... 11 Black Racer—Harmless ....................................................5 Northern Water Snake—Harmless ............................12 Ring-Necked Snake—Harmless ....................................6 Brown Water Snake—Harmless .................................12 Mud Snake—Harmless ....................................................6 Rough Green Snake—Harmless .................................12 -

A Guide to Missouri's Snakes
A GUIDE TO MISSOURI’S SNAKES MISSOURI DEPARTMENT OF CONSERVATION A Guide to Missouri’s Snakes by Jeffrey T. Briggler, herpetologist, and Tom R. Johnson, retired herpetologist, Missouri Department of Conservation Photographs by Jeffrey T. Briggler, Richard Daniel, Tom R. Johnson, and Jim Rathert Edited by Larry Archer Design by Susan Ferber Front cover: Eastern milksnake. Photo by Jim Rathert. mdc.mo.gov Copyright © 2017 by the Conservation Commission of the State of Missouri Published by the Missouri Department of Conservation PO Box 180, Jefferson City, Missouri 65102–0180 Equal opportunity to participate in and benefit from programs of the Missouri Depart- ment of Conservation is available to all individuals without regard to their race, color, religion, national origin, sex, ancestry, age, sexual orientation, veteran status, or disability. Questions should be directed to the Department of Conser- vation, PO Box 180, Jefferson City, MO 65102, 573-751-4115 (voice) or 800-735-2966 (TTY), or to Chief, Public Civil Rights, Office of Civil Rights, U.S. Department of the Interior, 1849 C Street, NW, Washington, D.C. 20240. GET TO KNOW MISSOURI’S SNAKES Snakes have generated more fear and misunderstanding than any other group of animals. Psychologists have proven that a fear of snakes (called ophidiophobia) is acquired; we are not born with it. Once people learn some of the interesting facts about snakes and discover that most of them are harmless and beneficial, their aversion may diminish. With patience and understanding, almost anyone can overcome a dread of snakes and actually enjoy studying them. One thing is certain — even people with a well-developed fear of snakes are curious about them.