Smooth Earth Snake N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tribological Analysis of Ventral Scale Structure in a Python Regius in Relation to Laser Textured Surfaces H
Tribological Analysis of Ventral Scale Structure in a Python Regius in Relation to Laser Textured Surfaces H. A. Abdel-Aal* Arts et Métiers ParisTech, LMPF-EA4106, Rue Saint Dominique BP 508, 51006, Châlons-en-Champagne, France *[email protected] M. El Mansori Ecole Nationale Supérieure d'Arts et Métiers, 151 Boulevard de l'Hôpital, 75013 PARIS, France ABSTRACT Laser Texturing is one of the leading technologies applied to modify surface topography. To date, however, a standardized procedure to generate deterministic textures is virtually non- existent. In nature, especially in squamata, there are many examples of deterministic structured textures that allow species to control friction and condition their tribological response for efficient function. In this work, we draw a comparison between industrial surfaces and reptilian surfaces. We chose the python regius species as a bio-analogue with a deterministic surface. We first study the structural make up of the ventral scales of the snake (both construction and metrology). We further compare the metrological features of the ventral scales to experimentally recommended performance indicators of industrial surfaces extracted from open literature. The results indicate the feasibility of engineering a Laser Textured Surface based on the reptilian ornamentation constructs. It is shown that the metrological features, key to efficient function of a rubbing deterministic surface, are already optimized in the reptile. We further show that optimization in reptilian surfaces is based on synchronizing surface form, textures and aspects to condition the frictional response. Mimicking reptilian surfaces, we argue, may form a design methodology potentially capable of generating advanced deterministic surface constructs capable of efficient tribological function. -

Species Identification of Shed Snake Skins in Taiwan and Adjacent Islands
Zoological Studies 56: 38 (2017) doi:10.6620/ZS.2017.56-38 Open Access Species Identification of Shed Snake Skins in Taiwan and Adjacent Islands Tein-Shun Tsai1,* and Jean-Jay Mao2 1Department of Biological Science and Technology, National Pingtung University of Science and Technology 1 Shuefu Road, Neipu, Pingtung 912, Taiwan 2Department of Forestry and Natural Resources, National Ilan University No.1, Sec. 1, Shennong Rd., Yilan City, Yilan County 260, Taiwan. E-mail: [email protected] (Received 28 August 2017; Accepted 25 November 2017; Published 19 December 2017; Communicated by Jian-Nan Liu) Tein-Shun Tsai and Jean-Jay Mao (2017) Shed snake skins have many applications for humans and other animals, and can provide much useful information to a field survey. When properly prepared and identified, a shed snake skin can be used as an important voucher; the morphological descriptions of the shed skins may be critical for taxonomic research, as well as studies of snake ecology and conservation. However, few convenient/ expeditious methods or techniques to identify shed snake skins in specific areas have been developed. In this study, we collected and examined a total of 1,260 shed skin samples - including 322 samples from neonates/ juveniles and 938 from subadults/adults - from 53 snake species in Taiwan and adjacent islands, and developed the first guide to identify them. To the naked eye or from scanned images, the sheds of almost all species could be identified if most of the shed was collected. The key features that aided in identification included the patterns on the sheds and scale morphology. -

Expanded Description of a Chinese Endemic Snake Opisthotropis Cheni (Serpentes: Colubridae: Natricinae)
Asian Herpetological Research 2010, 1(1): 57-60 DOI: 10.3724/SP.J.1245.2010.00057 Expanded Description of a Chinese Endemic Snake Opisthotropis cheni (Serpentes: Colubridae: Natricinae) LI Cao1, LIU Qin1 and GUO Peng 1, 2* 1 Department of Life Sciences and Food Engineering, Yibin University, Yibin 644000, Sichuan, China 2 Chendgu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China Abstract Based on seven newly-collected specimens, we provide an expanded description for the rare Chinese snake Opisthotropis cheni. The new specimens are consistent with the type series in scale counts and body dimensions. How- ever, two individuals lack yellow cross-bands that are apparent in the type specimens. A key to the ten Chinese species of Opisthotropis is provided. Keywords snake, Opisthotropis cheni, morphology, China 1. Introduction 070140, 071041, 071046-071050) (Table 1), collected from the Nanling National Nature Reserve in Ruyuan The genus Opisthotropis Gǘnther (1872) is comprised of County, Guangdong Province, China (Figure 1), were 17 currently recognized species, and is widely distributed morphologically examined in this work. The specimens in eastern, southern, and southeastern Asia (Uetz, 2010). were preserved in 8% formalin for initial fixation, and Members of this genus are all small aquatic snakes, later transferred to 75% ethanol. All specimens were de- mainly inhabiting rapidly flowing streams or small rivers. posited at Yibin University (YBU), Sichuan Province, Ten species of Opisthotropis occur in China (Zhao, China. 2006). Snout-vent length (SVL) and tail length (TL) were Zhao (1999) described Opisthotropis cheni based on measured using a meter ruler to the nearest millimeter, four specimens collected from Mt. -

By a Nine-Banded Armadillo (Dasypus Novemcinctus) in Santa Rosa National Park, Costa Rica
Edentata: in press Electronic version: ISSN 1852-9208 Print version: ISSN 1413-4411 http://www.xenarthrans.org FIELD NOTE Predation of a Central American coral snake (Micrurus nigrocinctus) by a nine-banded armadillo (Dasypus novemcinctus) in Santa Rosa National Park, Costa Rica Eduardo CarrilloA and Todd K. FullerB,1 A Instituto Internacional en Conservación y Manejo de Vida Silvestre, Universidad Nacional, Apdo. 1350, Heredia, Costa Rica. E-mail: [email protected] B Department of Environmental Conservation, University of Massachusetts, Amherst, Massachusetts 01003, USA. E-mail: [email protected] 1 Corresponding author Abstract We describe the manner in which a nine-banded armadillo (Dasypus novemcinctus) killed a Cen- tral American coral snake (Micrurus nigrocinctus) that it subsequently ate. The armadillo repeatedly ran towards, jumped, flipped over in mid-air, and landed on top of the snake with its back until the snake was dead. Keywords: armadillo, behavior, food, predation, snake Depredación de una serpiente de coral de América Central (Micrurus nigrocinctus) por un armadillo de nueve bandas (Dasypus novemcinctus) en el Parque Nacional Santa Rosa, Costa Rica Resumen En esta nota describimos la manera en que un armadillo de nueve bandas (Dasypus novemcinc- tus) mató a una serpiente de coral de América Central (Micrurus nigrocinctus) que posteriormente comió. El armadillo corrió varias veces hacia adelante, saltó, se dio vuelta en el aire y aterrizó sobre la serpiente con la espalda hasta que la serpiente estuvo muerta. Palabras clave: armadillo, comida, comportamiento, depredación, serpiente Nine-banded armadillos (Dasypus novemcinc- The ~4-kg nine-banded armadillo is distributed tus) feed mostly on arthropods such as beetles, ter- from the southeast and central United States to Uru- mites, and ants, but also consume bird eggs and guay and northern Argentina, Granada, Trinidad “unusual items” such as fruits, fungi, and small verte- and Tobago, and the Margarita Islands (Loughry brates (McBee & Baker, 1982; Wetzel, 1991; Carrillo et al., 2014). -
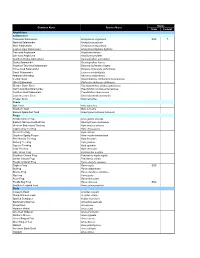
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

History and Function of Scale Microornamentation in Lacertid Lizards
JOURNALOFMORPHOLOGY252:145–169(2002) HistoryandFunctionofScaleMicroornamentation inLacertidLizards E.N.Arnold* NaturalHistoryMuseum,CromwellRoad,LondonSW75BD,UK ABSTRACTDifferencesinsurfacestructure(ober- mostfrequentlyinformsfromdryhabitatsorformsthat hautchen)ofbodyscalesoflacertidlizardsinvolvecell climbinvegetationawayfromtheground,situations size,shapeandsurfaceprofile,presenceorabsenceoffine wheredirtadhesionislessofaproblem.Microornamen- pitting,formofcellmargins,andtheoccurrenceoflongi- tationdifferencesinvolvingotherpartsofthebodyand tudinalridgesandpustularprojections.Phylogeneticin- othersquamategroupstendtocorroboratethisfunctional formationindicatesthattheprimitivepatterninvolved interpretation.Microornamentationfeaturescandevelop narrowstrap-shapedcells,withlowposteriorlyoverlap- onlineagesindifferentordersandappeartoactadditively pingedgesandrelativelysmoothsurfaces.Deviations inreducingshine.Insomecasesdifferentcombinations fromthisconditionproduceamoresculpturedsurfaceand maybeoptimalsolutionsinparticularenvironments,but havedevelopedmanytimes,althoughsubsequentovert lineageeffects,suchaslimitedreversibilityanddifferent reversalsareuncommon.Likevariationsinscaleshape, developmentalproclivities,mayalsobeimportantintheir differentpatternsofdorsalbodymicroornamentationap- peartoconferdifferentandconflictingperformancead- genesis.Thefinepitsoftenfoundoncellsurfacesare vantages.Theprimitivepatternmayreducefrictiondur- unconnectedwithshinereduction,astheyaresmaller inglocomotionandalsoenhancesdirtshedding,especially thanthewavelengthsofmostvisiblelight.J.Morphol. -

What Typical Population Density Could You Expect for the Species in a Hectare of Ideal Habitat?
SQUAMATES DENSITY - What typical population density could you expect for the species in a hectare of ideal habitat? Species Common Name Density Sauria Lizards Anguidae Anguid Lizards Ophisaurus attenuatus longicaudus Eastern Slender Glass Lizard >400 / ha; 4-111/ ha (Fitch 1989) Ophisaurus ventralis Eastern Glass Lizard Unk Lacertidae Wall Lizards Podarcis sicula Italian Wall Lizard Unk Phrynosomatidae Sceloporine Lizards Sceloporus undulatus hyacinthinus Northern Fence Lizard Unk Scincidae Skinks Eumeces a. anthracinus Northern Coal Skink Unk Eumeces fasciatus Common Five-lined Skink 85 / ha (Klemens 1993) Eumeces inexpectatus Southeastern Five-lined Skink Unk Eumeces laticeps Broad-headed Skink Unk Scincella lateralis Ground Skink 400-1500 / ha (Brooks 1967) Teiidae Whiptails Cnemidophorus s. sexlineatus Eastern Six-lined Racerunner 2.5 / 100 m2 (Mitchell 1994) Colubridae Colubrids Carphophis a. amoenus Eastern Worm Snake 60 - 120 / ha in KS (Clark 1970) Cemophora coccinea copei Northern Scarlet Snake Unk Clonophis kirtlandii Kirtland's Snake 19 along 0.6 km street (Minton 1972) Coluber c. constrictor Northern Black Racer 1-3 / ha (Ernst, pers. obs.); 3-7 / ha (Fitch 1963b) Diadophis p. punctatus Southern Ringneck Snake 719-1,849 / ha (Fitch 1975); > 100 / ha (Hulse, pers. obs.) Diadophis p. edwardsii Northern Ringneck Snake 719-1,849 / ha (Fitch 1975); > 100 / ha (Hulse, pers. obs.) Elaphe guttata Corn Snake less than 1 / 100 ha in KS (Fitch 1958a) Elaphe o. obsoleta Black Rat Snake 0.23 / ha MD (Stickel et al. 1980); 1 / ha in KS (Fitch 1963a) Farancia a. abacura Eastern Mud Snake about 150 / km (Hellman and Telford 1956) Farancia e. erytrogramma Common Rainbow Snake 8 in 30 m (Mount 1975); 20 in 4.1 ha (Richmond 1945) Heterodon platirhinos Eastern Hog-nosed Snake 2.1 / ha (Platt 1969); 4.8 / ha in VA (Scott 1986) Lampropeltis calligaster Mole Kingsnake 1 / 2.6 ha (Ernst and Barbour 1989) rhombomaculata Lampropeltis g. -

Check List 17 (1): 27–38
17 1 ANNOTATED LIST OF SPECIES Check List 17 (1): 27–38 https://doi.org/10.15560/17.1.27 A herpetological survey of Edith L. Moore Nature Sanctuary Dillon Jones1, Bethany Foshee2, Lee Fitzgerald1 1 Biodiversity Research and Teaching Collections, Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, USA. 2 Houston Audubon, 440 Wilchester Blvd. Houston, TX 77079 USA. Corresponding author: Dillon Jones, [email protected] Abstract Urban herpetology deals with the interaction of amphibians and reptiles with each other and their environment in an ur- ban setting. As such, well-preserved natural areas within urban environments can be important tools for conservation. Edith L. Moore Nature Sanctuary is an 18-acre wooded sanctuary located west of downtown Houston, Texas and is the headquarters to Houston Audubon Society. This study compared iNaturalist data with results from visual encounter surveys and aquatic funnel traps. Results from these two sources showed 24 species belonging to 12 families and 17 genera of herpetofauna inhabit the property. However, several species common in surrounding areas were absent. Combination of data from community science and traditional survey methods allowed us to better highlight herpe- tofauna present in the park besides also identifying species that may be of management concern for Edith L. Moore. Keywords Community science, iNaturalist, urban herpetology Academic editor: Luisa Diele-Viegas | Received 27 August 2020 | Accepted 16 November 2020 | Published 6 January 2021 Citation: Jones D, Foshee B, Fitzgerald L (2021) A herpetology survey of Edith L. Moore Nature Sanctuary. Check List 17 (1): 27–28. https://doi. -

Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Gulsby Kennesaw State University
Kennesaw State University DigitalCommons@Kennesaw State University Department of Ecology, Evolution, and Organismal Master of Science in Integrative Biology Theses Biology Summer 7-25-2019 Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Gulsby Kennesaw State University Follow this and additional works at: https://digitalcommons.kennesaw.edu/integrbiol_etd Part of the Integrative Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Gulsby, Miranda, "Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia" (2019). Master of Science in Integrative Biology Theses. 48. https://digitalcommons.kennesaw.edu/integrbiol_etd/48 This Thesis is brought to you for free and open access by the Department of Ecology, Evolution, and Organismal Biology at DigitalCommons@Kennesaw State University. It has been accepted for inclusion in Master of Science in Integrative Biology Theses by an authorized administrator of DigitalCommons@Kennesaw State University. For more information, please contact [email protected]. Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Louise Gulsby A Thesis Presented in Partial Fulfillment of Requirements of the Master of Science in Integrative Biology for the Department of Evolution, Ecology, and Organismal Biology Kennesaw State University 1000 Chastain Road Kennesaw, Ga 30144 July 2019 Major Advisor: Thomas McElroy, Ph. D. Committee Members: Joel McNeal, Ph. -

Indigenous and Established Herpetofauna of Northwest Louisiana
Indigenous and Established Herpetofauna of Northwest Louisiana Non-venomous Snakes (25 Species) For more info: Buttermilk Racer Coluber constrictor anthicus 318-773-9393 Eastern Coachwhip Coluber flagellum flagellum www.learnaboutcritters.org Prairie Kingsnake Lampropeltis calligaster [email protected] Speckled Kingsnake Lampropeltis holbrooki www.facebook.com/learnaboutcritters Western Milksnake Lampropeltis gentilis Northern Rough Greensnake Opheodrys aestivus aestivus Alligator (1 species) Western Ratsnake Pantherophis obsoletus American Alligator Alligator mississippiensis Slowinski’s Cornsnake † Pantherophis slowinskii Flat-headed Snake † Tantilla gracilis Western Wormsnake † Carphophis vermis Lizards (10 Species) Mississippi Ring-necked Snake Diadophis punctatus stictogenys Western Mudsnake Farancia abacura reinwardtii Northern Green Anole Anolis carolinensis Eastern Hog-nosed Snake Heterodon platirhinos Prairie Lizard Sceloporus consobrinus Mississippi Green Watersnake Nerodia cyclopion Southern Coal Skink † Plestiodon anthracinus pluvialis Plain-bellied Watersnake Nerodia erythrogaster Common Five-lined Skink Plestiodon fasciatus Broad-banded Watersnake Nerodia fasciata confluens Broad-headed Skink Plestiodon laticeps Graham's Crayfish Snake Regina grahamii Southern Prairie Skink † Plestiodon septentrionalis obtusirostris Gulf Swampsnake Liodytes rigida sinicola Little Brown Skink Scincella lateralis Dekay’s Brownsnake Storeria dekayi Eastern Six-lined Racerunner Aspidoscelis sexlineata sexlineata Red-bellied Snake -

Significant New Records of Amphibians and Reptiles from Georgia, USA
GEOGRAPHIC DISTRIBUTION 597 Herpetological Review, 2015, 46(4), 597–601. © 2015 by Society for the Study of Amphibians and Reptiles Significant New Records of Amphibians and Reptiles from Georgia, USA Distributional maps found in Amphibians and Reptiles of records for a variety of amphibian and reptile species in Georgia. Georgia (Jensen et al. 2008), along with subsequent geographical All records below were verified by David Bechler (VSU), Nikole distribution notes published in Herpetological Review, serve Castleberry (GMNH), David Laurencio (AUM), Lance McBrayer as essential references for county-level occurrence data for (GSU), and David Steen (SRSU), and datum used was WGS84. herpetofauna in Georgia. Collectively, these resources aid Standard English names follow Crother (2012). biologists by helping to identify distributional gaps for which to target survey efforts. Herein we report newly documented county CAUDATA — SALAMANDERS DIRK J. STEVENSON AMBYSTOMA OPACUM (Marbled Salamander). CALHOUN CO.: CHRISTOPHER L. JENKINS 7.8 km W Leary (31.488749°N, 84.595917°W). 18 October 2014. D. KEVIN M. STOHLGREN Stevenson. GMNH 50875. LOWNDES CO.: Langdale Park, Valdosta The Orianne Society, 100 Phoenix Road, Athens, (30.878524°N, 83.317114°W). 3 April 1998. J. Evans. VSU C0015. Georgia 30605, USA First Georgia record for the Suwannee River drainage. MURRAY JOHN B. JENSEN* CO.: Conasauga Natural Area (34.845116°N, 84.848180°W). 12 Georgia Department of Natural Resources, 116 Rum November 2013. N. Klaus and C. Muise. GMNH 50548. Creek Drive, Forsyth, Georgia 31029, USA DAVID L. BECHLER Department of Biology, Valdosta State University, Valdosta, AMBYSTOMA TALPOIDEUM (Mole Salamander). BERRIEN CO.: Georgia 31602, USA St. -

Reptiles in Arkansas
Terrestrial Reptile Report Carphophis amoenus Co mmon Wor msnake Class: Reptilia Order: Serpentes Family: Colubridae Priority Score: 19 out of 100 Population Trend: Unknown Global Rank: G5 — Secure State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Arkansas Valley Ouachita Mountains South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plain Carphophis amoenus Common Wormsnake 1079 Terrestrial Reptile Report Habitat Map Habitats Weight Crowley's Ridge Loess Slope Forest Obligate Lower Mississippi Flatwoods Woodland and Forest Suitable Problems Faced KNOWN PROBLEM: Habitat loss due to conversion Threat: Habitat destruction or to agriculture. conversion Source: Agricultural practices KNOWN PROBLEM: Habitat loss due to forestry Threat: Habitat destruction or practices. conversion Source: Forestry activities Data Gaps/Research Needs Genetic analyses comparing Arkansas populations with populations east of the Mississippi River and the Western worm snake. Conservation Actions Importance Category More data are needed to determine conservation actions. Monitoring Strategies More information is needed to develop a monitoring strategy. Carphophis amoenus Common Wormsnake 1080 Terrestrial Reptile Report Comments Trauth and others (2004) summarized the literature and biology of this snake. In April 2005, two new geographic distribution records were collected in Loess Slope Forest habitat within St. Francis National Forest, south of the Mariana