Contents Herpetological Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Very European Tale – Britain Still Has Only Three Snake Species, but Its Grass Snake Is Now Assigned to Another Species (Natrix Helvetica)
SHORT COMMUNICATION The Herpetological Bulletin 141, 2017: 44-45 A very European tale – Britain still has only three snake species, but its grass snake is now assigned to another species (Natrix helvetica) UWE FRITZ1* & CAROLIN KINDLER1 1Senckenberg Natural History Collections Dresden, Museum of Zoology, A. B. Meyer Building, 01109 Dresden, Germany *Corresponding author Email: [email protected] ollowing several investigations of the phylogeography and systematics of grass snakes (Fritz et al., 2012; FKindler et al., 2013, 2014; Pokrant et al., 2016), we published a further detailed study on this topic in August (Kindler et al., 2017). Our new investigation revealed that only very limited gene flow occurs between western barred grass snakes and eastern common grass snakes. Consequently, we concluded that the barred grass snake (Fig. 1), previously a subspecies, should be elevated to a full species. August being the ‘silly season’ for news stories led the local media, including the highly respected BBC, to claim that Britain has now an additional snake species, i.e. four instead of three species – the northern viper (Vipera Figure 1. Young N. helvetica showing the distinctive lateral bars berus), the smooth snake (Coronella austriaca) as well as from which the species common name the ‘barred grass snake’ is derived (photo: © Jason Steel) two species of grass snake, the common grass snake (Natrix natrix) and the newly recognised barred grass snake (Natrix helvetica). findings. However, some southern populations identified This upheaval resulted from a complete misunderstanding by Thorpe with barred grass snakes, for instance from of a press release by the Senckenberg Institution. The press northern Italy, turned out to be distinct from N. -

Population and Ecological Characteristics of the Dice Snake, Natrix Tessellata
Turkish Journal of Zoology Turk J Zool (2019) 43: 657-664 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Short Communication doi:10.3906/zoo-1811-8 Population and ecological characteristics of the dice snake, Natrix tessellata (Laurenti, 1768), in lower portions of the Vrbanja River (Republic of Srpska, Bosnia and Herzegovina) 1, 2 1 2 Goran ŠUKALO *, Sonja NIKOLIĆ , Dejan DMITROVIĆ , Ljiljana TOMOVIĆ 1 Faculty of Natural Sciences and Mathematics, University of Banja Luka, Banja Luka, Republic of Srpska, Bosnia and Herzegovina 2 Institute of Zoology, Faculty of Biology, University of Belgrade, Belgrade, Serbia Received: 06.11.2018 Accepted/Published Online: 12.09.2019 Final Version: 01.11.2019 Abstract: Despite their comparative richness and accessibility in the Republic of Srpska and in Bosnia and Herzegovina in general, population studies of reptiles have not been performed in Srpska until recently. For example, one of the most common snake species in this area is the dice snake; nevertheless, previous studies have only reported its distribution. The aim of the present study was to analyze characteristics of the dice snake population along the Vrbanja River. Animals were processed during 2011 throughout their activity period. In total, 199 individuals of all ages were collected. We observed substantial differences in numbers of animals captured in different habitat types classified according to the level of anthropogenic influence. Unexpectedly, the largest number of snakes was captured in the zone with the highest anthropogenic influence, while the smallest number was observed in the zone with no anthropogenic pressures. The above is probably connected with the observed greater number of their most common prey, as well as the absence of raptors in areas with human impact. -

The Journey of Life of the Tiger-Striped Leaf Frog Callimedusa Tomopterna (Cope, 1868): Notes of Sexual Behaviour, Nesting and Reproduction in the Brazilian Amazon
Herpetology Notes, volume 11: 531-538 (2018) (published online on 25 July 2018) The journey of life of the Tiger-striped Leaf Frog Callimedusa tomopterna (Cope, 1868): Notes of sexual behaviour, nesting and reproduction in the Brazilian Amazon Thainá Najar1,2 and Lucas Ferrante2,3,* The Tiger-striped Leaf Frog Callimedusa tomopterna 2000; Venâncio & Melo-Sampaio, 2010; Downie et al, belongs to the family Phyllomedusidae, which is 2013; Dias et al. 2017). constituted by 63 described species distributed in In 1975, Lescure described the nests and development eight genera, Agalychnis, Callimedusa, Cruziohyla, of tadpoles to C. tomopterna, based only on spawns that Hylomantis, Phasmahyla, Phrynomedusa, he had found around the permanent ponds in the French Phyllomedusa, and Pithecopus (Duellman, 2016; Guiana. However, the author mentions a variation in the Frost, 2017). The reproductive aspects reported for the number of eggs for some spawns and the use of more than species of this family are marked by the uniqueness of one leaf for confection in some nests (Lescure, 1975). egg deposition, placed on green leaves hanging under The nests described by Lescure in 1975 are probably standing water, where the tadpoles will complete their from Phyllomedusa vailantii as reported by Lescure et development (Haddad & Sazima, 1992; Pombal & al. (1995). The number of eggs in the spawns reported Haddad, 1992; Haddad & Prado, 2005). However, by Lescure (1975) diverge from that described by other exist exceptions, some species in the genus Cruziohyla, authors such as Neckel-Oliveira & Wachlevski, (2004) Phasmahylas and Prhynomedusa, besides the species and Lima et al. (2012). In addition, the use of more than of the genus Agalychnis and Pithecopus of clade one leaf for confection in the nest mentioned by Lescure megacephalus that lay their eggs in lotic environments (1975), are characteristic of other species belonging to (Haddad & Prado, 2005; Faivovich et al. -

Management Plan National Park Prespa in Albania
2014-2024 Plani i Menaxhimit të Parkut Kombëtar të Prespës në Shqipëri PPLLAANNII II MMEENNAAXXHHIIMMIITT II PPAARRKKUUTT KKOOMMBBËËTTAARR TTËË PPRREESSPPËËSS NNËË SSHHQQIIPPËËRRII 22001144--22002244 1 Plani i Menaxhimit të Parkut Kombëtar të Prespës në Shqipëri 2013-2023 SHKURTIME ALL Monedha Lek a.s.l. Mbi nivelin e detit BCA Konsulent për ruajtjen e biodiversitetit BMZ Ministria Federale për Kooperimin Ekonomik dhe Zhvillimin, Gjermani CDM Mekanizmi për Zhvillimin e Pastër Corg Karbon organik DCM Vendim i Këshillit të Ministrave DFS Drejtoria e Shërbimit Pyjor, Korca DGFP Drejtoria e Pyjeve dhe Kullotave DTL Zevendes Drejtues i Ekipit EUNIS Sistemi i Informacionit të Natyrës së Bashkimit Evropian GEF Faciliteti Global për Mjedisin GFA Grupi Konsulent GFA, Gjermani GNP Parku Kombëtar i Galicicës GO Organizata Qeveritare GTZ/GIZ Agjensia Gjermane për Bashkëpunim Teknik (Sot quhet GIZ) FAO Organizata e Kombeve të Bashkuara për Ushqimin dhe Bujqësinë IUCN Bashkimi Ndërkombëtar për Mbrojtjen e Natyrës FUA Shoqata e Përdoruesve të Pyjeve, Prespë KfW Banka Gjermane për Zhvillim LMS Vende për monitorimin afatgjatë LSU Njësi blegtorale MC Komiteti i Menaxhimit të Parkut Kombëtar të Prespës në Shqipëri METT Mjeti për Gjurmimin e Efektivitetit të Menaxhimit MoE Ministria e Mjedisit, Shqipëri MP Plan menaxhimi NGO Organizata jo-fitimprurëse NP Park Kombëtar NPA Administrata e Parkut Kombëtar NPD Drejtor i Parkut Kombëtar (aktualisht shef i sektorit të PK të Prespës të Drejtorisë së Shërbimit Pyjor, Korçë) PNP Parku Kombëtar i Prespës ÖBF AG Korporata -

Khalladi-Bpp Anexes-Arabic.Pdf
Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 107 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 108 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 109 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 110 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 111 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 112 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 113 The IUCN Red List Categories and Criteria are intended to be an easily and widely understood system for classifying species at high risk of global extinction. The IUCN Red List is categorized in the following Categories: • Extinct (EX): A taxon is Extinct when there is no reasonable doubt that the last individual has died. A taxon is presumed Extinct when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. Surveys should be over a time frame appropriate to the taxon’s life cycle and life form. Khalladi Windfarm and Power Line Projects 114 Biodiversity Protection Plan, July 2015 • Extinct in the Wild (EW): A taxon is Extinct in the Wild when it is known only to survive in cultivation, in captivity or as a naturalized population (or populations) well outside the past range. A taxon is presumed Extinct in the Wild when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. -
B I R D W a T C H I N G •
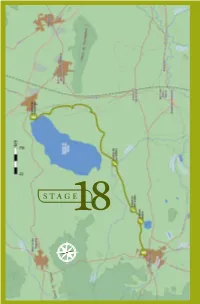
18 . F UENTE DE PIEDRA - CAMPILLOS STAGE18 E S N O 158 BIRDWATCHING • GR-249 Great Path of Malaga F UENTE DE PIEDRA - CAMPILLOS 18 . STAGE 18 Fuente de Piedra - Campillos L O C A T I O N he José Antonio Valverde Visitor´s Centre at the TReserva Natural de la Laguna de Fuente de Piedra is the starting point of Stage 18. Taking the direction south around the taken up mainly by olive trees and grain. eastern side of the salt water lagoon This type of environment will continue to you will be walking through farmland the end of this stage and it determines until the end of this stage in Campillos the species of birds which can be seen village. The 15, 7 km of Stage 18 will here. You will be crossing a stream and allow you to discover this wetland well then walking along the two lakes which known at national level in Spain, and will make your Stage 18 bird list fill cultivated farmland which creates a up with highly desirable species. The steppe-like environment. combination of wetland and steppe creates very valuable habitats with DESCRIPTION a rare composition of taxa unique at European level. ABOUT THE BIRDLIFE: Stage 18 begins at the northern tip HIGHLIGHTED SPECIES of the lagoon where you take direction Neither the length, diffi culty level or south through agricultural environment, elevation gain of this stage is particularly DID YOU KNOW? ecilio Garcia de la Leña, in Conversation 9th of “Historical Conversations of Malaga” published by Cristóbal Medina Conde (1726-1798) and entitled «About Animal Kingdom of Malaga and some Places in its Bishopric», Ccomments: «…In some lagoons, along sea shores and river banks some large and beautiful birds breed, called Flamingos and Phoenicopteros according to the ancients...», after a description of the bird´s anatomy he adds: «The Romans appreciated the bird greatly, especially its tongue which was served as an exquisite dish…». -

Raymond T. Hoser
32 Australasian Journal of Herpetology Australasian Journal of herpetology 11:32-50. Published 8 April 2012. ISSN 1836-5698 (Print) ISSN 1836-5779 (Online) THE DESCRIPTION OF A NEW GENUS OF WEST AUSTRALIAN SNAKE AND EIGHT NEW TAXA IN THE GENERA PSEUDONAJA GUNTHER, 1858, OXYURANUS KINGHORN, 1923 AND PANACEDECHIS WELLS AND WELLINGTON, 1985 (SERPENTES: ELAPIDAE) RAYMOND T. HOSER 488 Park Road, Park Orchards, Victoria, 3134, Australia. Phone: +61 3 9812 3322 Fax: 9812 3355 E-mail: [email protected] Submitted 20 March 2012, Accepted 30 March 2012, Published 8 April 2012. ABSTRACT This paper defines and names new taxa from Australasia. The taxon Denisonia fasciata Rosen 1905, placed most recently by most authors in the genus Suta, is formally removed from that genus and placed in a monotypic genus formally named and described herein. Other taxa formally named and described for the first time include subspecies of the following; the broadly recognized species Pseudonaja textilis (known as the Eastern Brown Snake), P. guttata (Speckled Brown Snake) and P. affinis (Dugite), Oxyuranus scutellatus (Taipan) from Irian Jaya and western Papua as well as a second subspecies from north-west Australia and a hitherto unnamed subspecies of Panacedechis papuanus (Papuan Blacksnake) from the same general region. The newly named taxa are: Hulimkai gen. nov., Pseudonaja textilis cliveevatti subsp. nov., Pseudonaja textilis leswilliamsi subsp. nov., Pseudonaja textilis rollinsoni subsp. nov., Pseudonaja textilis jackyhoserae subsp. nov., Pseudonaja guttata -

A Global Overview of Protected Areas on the World Heritage List of Particular Importance for Biodiversity
A GLOBAL OVERVIEW OF PROTECTED AREAS ON THE WORLD HERITAGE LIST OF PARTICULAR IMPORTANCE FOR BIODIVERSITY A contribution to the Global Theme Study of World Heritage Natural Sites Text and Tables compiled by Gemma Smith and Janina Jakubowska Maps compiled by Ian May UNEP World Conservation Monitoring Centre Cambridge, UK November 2000 Disclaimer: The contents of this report and associated maps do not necessarily reflect the views or policies of UNEP-WCMC or contributory organisations. The designations employed and the presentations do not imply the expressions of any opinion whatsoever on the part of UNEP-WCMC or contributory organisations concerning the legal status of any country, territory, city or area or its authority, or concerning the delimitation of its frontiers or boundaries. TABLE OF CONTENTS EXECUTIVE SUMMARY INTRODUCTION 1.0 OVERVIEW......................................................................................................................................................1 2.0 ISSUES TO CONSIDER....................................................................................................................................1 3.0 WHAT IS BIODIVERSITY?..............................................................................................................................2 4.0 ASSESSMENT METHODOLOGY......................................................................................................................3 5.0 CURRENT WORLD HERITAGE SITES............................................................................................................4 -

Indigenous Reptiles
Reptiles Sylvain Ursenbacher info fauna & NLU, Universität Basel pdf can be found: www.ursenbacher.com/teaching/Reptilien_UNIBE_2020.pdf Reptilia: Crocodiles Reptilia: Tuataras Reptilia: turtles Rep2lia: Squamata: snakes Rep2lia: Squamata: amphisbaenians Rep2lia: Squamata: lizards Phylogeny Tetrapoda Synapsida Amniota Lepidosauria Squamata Sauropsida Anapsida Archosauria H4 Phylogeny H5 Chiari et al. BMC Biology 2012, 10:65 Amphibians – reptiles - differences Amphibians Reptiles numerous glands, generally wet, without or with limited number skin without scales of glands, dry, with scales most of them in water, no links with water, reproduction larval stage without a larval stage most of them in water, packed in not in water, hard shell eggs tranparent jelly (leathery or with calk) passive transmission of venom, some species with active venom venom toxic skin as passive protection injection Generally in humide and shady Generally dry and warm habitats areas, nearby or directly in habitats, away from aquatic aquatic habitats habitats no or limited seasonal large seasonal movements migration movements, limited traffic inducing big traffic problems problems H6 First reptiles • first reptiles: about 320-310 millions years ago • embryo is protected against dehydration • ≈ 305 millions years ago: a dryer period ➜ new habitats for reptiles • Mesozoic (252-66 mya): “Age of Reptiles” • large disparition of species: ≈ 252 and 65 millions years ago H7 Mesozoic Quick systematic overview total species CH species (oct 2017) Order Crocodylia (crocodiles) -

Cleaning the Linnean Stable of Names for Grass Snakes (Natrix Astreptophora, N
70 (4): 621– 665 © Senckenberg Gesellschaft für Naturforschung, 2020. 2020 The Fifth Labour of Heracles: Cleaning the Linnean stable of names for grass snakes (Natrix astreptophora, N. helvetica, N. natrix sensu stricto) Uwe Fritz 1 & Josef Friedrich Schmidtler 2 1 Museum of Zoology, Senckenberg Dresden, A. B. Meyer Building, 01109 Dresden, Germany; [email protected] — 2 Liebenstein- straße 9A, 81243 Munchen, Germany; [email protected] Submitted July 29, 2020. Accepted October 29, 2020. Published online at www.senckenberg.de/vertebrate-zoology on November 12, 2020. Published in print Q4/2020. Editor in charge: Ralf Britz Abstract We scrutinize scientifc names erected for or referred to Natrix astreptophora (Seoane, 1884), Natrix helvetica (Lacepède, 1789), and Natrix natrix (Linnaeus, 1758). As far as possible, we provide synonymies for the individual subspecies of each species, identify each name with one of the mtDNA lineages or nuclear genomic clusters within these taxa, and clarify the whereabouts of type material. In addi tion, we feature homonyms and names erroneously identifed with grass snakes. For Natrix astreptophora (Seoane, 1884), we recognize a second subspecies from North Africa under the name Natrix astreptophora algerica (Hecht, 1930). The nominotypical subspecies occurs in the European part of the distribution range (Iberian Peninsula, adjacent France). Within Natrix helvetica (Lacepède, 1789), we recognize four subspecies. The nominotypical subspecies occurs in the northern distribution range, Natrix helvetica sicula (Cuvier, 1829) in Sicily, mainland Italy and adjacent regions, Natrix helvetica cetti Gené, 1839 on Sardinia, and Natrix helvetica corsa (Hecht, 1930) on Corsica. However, the validity of the latter subspecies is questionable. -

Taxonomy of the Genus Pseudonaja (Reptilia: Elapidae) in Australia
AUSTRALIAN BIODIVERSITY RECORD ________________________________________________________ 2002 (No 7) ISSN 1325-2992 March, 2002 ________________________________________________________ Taxonomy of the Genus Pseudonaja (Reptilia: Elapidae) in Australia. by Richard W. Wells “Shiralee”, Major West Road, Cowra, New South Wales, Australia The clear morphological differences that exist within the genus as previously considered strongly indicate that it is a polyphyletic assemblage. Accordingly, I have taken the step of formally proposing the fragmentation of Pseudonaja. In this work I have decided to restrict the genus Pseudonaja to the Pseudonaja nuchalis complex. Additionally, I herein formally resurrect from synonymy the generic name Euprepiosoma Fitzinger, 1860 for the textilis group of species, erect a new generic name (Placidaserpens gen. nov.) for the snakes previously regarded as Pseudonaja guttata, erect a new generic name (Notopseudonaja gen. nov.) for the group of species previously regarded as the Pseudonaja modesta complex, and erect a new generic name (Dugitophis gen. nov.) for snakes previously regarded as the Pseudonaja affinis complex. Genus Pseudonaja Gunther, 1858 The Pseudonaja nuchalis Complex It is usually reported that Pseudonaja nuchalis occurs across most of northern, central and western Australia, ranging from Cape York Peninsula, in the north-east, through western, southern and south-eastern Queensland, far western New South Wales, north-western Victoria, and most of South Australia, Northern Territory and Western Australia. However, this distribution pattern is now known to actually represents several different species all regarded by most authorities for convenience as the single highly variable species, 'Pseudonaja nuchalis'. As usually defined, this actually is a highly variable and therefore confusing group of species to identify and it is not all surprising that there has been difficulty in breaking up the group. -

Peptides to Tackle Leishmaniasis: Current Status and Future Directions
International Journal of Molecular Sciences Review Peptides to Tackle Leishmaniasis: Current Status and Future Directions Alberto A. Robles-Loaiza 1, Edgar A. Pinos-Tamayo 1, Bruno Mendes 2,Cátia Teixeira 3 , Cláudia Alves 3 , Paula Gomes 3 and José R. Almeida 1,* 1 Biomolecules Discovery Group, Universidad Regional Amazónica Ikiam, Tena 150150, Ecuador; [email protected] (A.A.R.-L.); [email protected] (E.A.P.-T.) 2 Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas (UNICAMP), Campinas 13083-862, Brazil; [email protected] 3 LAQV-REQUIMTE, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade do Porto, 4169-007 Porto, Portugal; [email protected] (C.T.); [email protected] (C.A.); [email protected] (P.G.) * Correspondence: [email protected] Abstract: Peptide-based drugs are an attractive class of therapeutic agents, recently recognized by the pharmaceutical industry. These molecules are currently being used in the development of innovative therapies for diverse health conditions, including tropical diseases such as leishmaniasis. Despite its socioeconomic influence on public health, leishmaniasis remains long-neglected and categorized as a poverty-related disease, with limited treatment options. Peptides with antileishmanial effects encountered to date are a structurally heterogeneous group, which can be found in different natural sources—amphibians, reptiles, insects, bacteria, marine organisms, mammals, plants, and others—or inspired by natural toxins or proteins. This review details the biochemical and structural characteris- Citation: Robles-Loaiza, A.A.; tics of over one hundred peptides and their potential use as molecular frameworks for the design of Pinos-Tamayo, E.A.; Mendes, B.; antileishmanial drug leads.