Expanded Description of a Chinese Endemic Snake Opisthotropis Cheni (Serpentes: Colubridae: Natricinae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Species of the Genus Opisthotropis Günther, 1872 from Northern Laos (Squamata: Natricidae)
Zootaxa 3774 (2): 165–182 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3774.2.4 http://zoobank.org/urn:lsid:zoobank.org:pub:933179AB-E8DB-4785-9F79-E0D051E1E398 A new species of the genus Opisthotropis Günther, 1872 from northern Laos (Squamata: Natricidae) ALEXANDRE TEYNIÉ1, ANNE LOTTIER1, PATRICK DAVID*2, TRUONG QUANG NGUYEN3 & GERNOT VOGEL4 1 Société d'Histoire Naturelle Alcide d’Orbigny, 57 rue de Gergovie, F-63170 Aubière, France. E-mail: [email protected] 2 Reptiles & Amphibiens, UMR 7205 OSEB, Département Systématique et Évolution, CP 30, Muséum National d’Histoire Naturelle, 57 rue Cuvier, 75231 Paris Cedex 05, France. E-mail: [email protected] 3 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam Current address: Department of Terrestrial Ecology, Zoological Institute, University of Cologne, Zülpicher Strasse 47b, D-50674 Cologne, Germany. E-mail: [email protected] 4 Society for Southeast Asian Herpetology, Im Sand 3, D-69115 Heidelberg, Germany. E-mail: [email protected] * corresponding author Abstract Two specimens, a male and a female, of the genus Opisthotropis Günther, 1872 were collected in a karst formation of northern Louangphabang (or Luang Prabang) Province, North Laos. These specimens are assigned to the genus Opisthotropis on the basis of their morphology, dentition and cephalic scalation. However, they differ from -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

NHBSS 061 1G Hikida Fieldg
Book Review N$7+IST. BULL. S,$0 SOC. 61(1): 41–51, 2015 A Field Guide to the Reptiles of Thailand by Tanya Chan-ard, John W. K. Parr and Jarujin Nabhitabhata. Oxford University Press, New York, 2015. 344 pp. paper. ISBN: 9780199736492. 7KDLUHSWLOHVZHUHÀUVWH[WHQVLYHO\VWXGLHGE\WZRJUHDWKHUSHWRORJLVWV0DOFROP$UWKXU 6PLWKDQG(GZDUG+DUULVRQ7D\ORU7KHLUFRQWULEXWLRQVZHUHSXEOLVKHGDV6MITH (1931, 1935, 1943) and TAYLOR 5HFHQWO\RWKHUERRNVDERXWUHSWLOHVDQGDPSKLELDQV LQ7KDLODQGZHUHSXEOLVKHG HJ&HAN-ARD ET AL., 1999: COX ET AL DVZHOODVPDQ\ SDSHUV+RZHYHUWKHVHERRNVZHUHWD[RQRPLFVWXGLHVDQGQRWJXLGHVIRURUGLQDU\SHRSOH7ZR DGGLWLRQDOÀHOGJXLGHERRNVRQUHSWLOHVRUDPSKLELDQVDQGUHSWLOHVKDYHDOVREHHQSXEOLVKHG 0ANTHEY & GROSSMANN, 1997; DAS EXWWKHVHERRNVFRYHURQO\DSDUWRIWKHIDXQD The book under review is very well prepared and will help us know Thai reptiles better. 2QHRIWKHDXWKRUV-DUXMLQ1DEKLWDEKDWDZDVP\ROGIULHQGIRUPHUO\WKH'LUHFWRURI1DWXUDO +LVWRU\0XVHXPWKH1DWLRQDO6FLHQFH0XVHXP7KDLODQG+HZDVDQH[FHOOHQWQDWXUDOLVW DQGKDGH[WHQVLYHNQRZOHGJHDERXW7KDLDQLPDOVHVSHFLDOO\DPSKLELDQVDQGUHSWLOHV,Q ZHYLVLWHG.KDR6RL'DR:LOGOLIH6DQFWXDU\WRVXUYH\KHUSHWRIDXQD+HDGYLVHGXV WRGLJTXLFNO\DURXQGWKHUH:HFROOHFWHGIRXUVSHFLPHQVRIDibamusZKLFKZHGHVFULEHG DVDQHZVSHFLHVDibamus somsaki +ONDA ET AL 1RZ,DPYHU\JODGWRNQRZWKDW WKLVERRNZDVSXEOLVKHGE\KLPDQGKLVFROOHDJXHV8QIRUWXQDWHO\KHSDVVHGDZD\LQ +LVXQWLPHO\GHDWKPD\KDYHGHOD\HGWKHSXEOLFDWLRQRIWKLVERRN7KHERRNLQFOXGHVQHDUO\ DOOQDWLYHUHSWLOHV PRUHWKDQVSHFLHV LQ7KDLODQGDQGPRVWSLFWXUHVZHUHGUDZQZLWK H[FHOOHQWGHWDLO,WLVDYHU\JRRGÀHOGJXLGHIRULGHQWLÀFDWLRQRI7KDLUHSWLOHVIRUVWXGHQWV -

CITY of ST. CATHARINES a By-Law to Amend By-Law No. 95-212 Entitled
' CITY OF ST. CATHARINES A By-law to amend By-law No. 95-212 entitled "A By-law to regulate the keeping of animals." AND WHEREAS by giving the required public notice and holding a public meeting, the City of St. Catharines has complied with the statutory notices required , and notice of the said by-law was posted to the City of St. Catharines website on September 10, 2013, and the public meeting was held on September 23, 2013; WHEREAS section 11 (2) of the Municipal Act provides authority for lower-tier municipalities to pass by-laws respecting health, safety and well-being of persons; AND WHEREAS section 103 of the Municipal Act provides authority for municipalities to pass by-laws to regulate or prohibit with respect to animals being at large; AND NOW THEREFORE THE COUNCIL OF THE CORPORATION OF THE CITY OF ST. CATHARINES enacts as follows: 1. That By-law No. 95-212, as amended, is hereby further amended by deleting the words "Any venomous Reptilia (such as venomous snakes and lizards)" in Schedule "A" and Schedule "B" thereof and replacing with the following: "All Reptilia as follows: (a) all Helodermatidae (e.g. gila monster and Mexican bearded lizard); (b) all front-fanged venomous snakes, even if devenomized, including, but not limited to: (i) all Viperidae (e.g. viper, pit viper), (ii) all Elapidae (e.g. cobra, mamba, krait, coral snake), (iii) all Atractaspididae (e.g. African burrowing asp), (iv) all Hydrophiidae (e.g. sea snake), and 2 (v) all Laticaudidae (e.g. sea krait); (c) all venomous, mid- or rear-fanged , Duvernoy-glanded -

Scaling Up: the Contemporary Reptile Pet Market in Japan
S H O R T R E P O R T SCALING UP: THE CONTEMPORARY REPTILE PET MARKET IN JAPAN CHINESE WATER DRAGON / © J. JANSSEN J. © / DRAGON WATER CHINESE 64 TRAFFIC Bulletin 9RO1R SS H H O O R R T T R R E E P P O O R R T T 5HSRUWE\.HLNR:DNDR -RUGL-DQVVHQDQG 6HUHQH&KQJ TRAFFIC Bulletin9RO1R S H O R T R E P O R T ,ඇඍඋඈൽඎർඍංඈඇ 0ൾඍඁඈൽඌ he reptile pet industry has been scrutinised by • Market survey the international conservation community for In order to investigate the reptiles for sale in pet shops its role in the trade of a wide range of species, and expos in Japan, TRAFFIC investigators carried many of which are threatened by collection out surveys of eight outlets in Tokyo, six in Kanagawa for trade (Herrel and van der Meijden, 2014; Prefecture, and two in Osaka Prefecture in February TAuliya et al., ,QWHUPVRIPRQHWDU\YDOXH-DSDQ 2017. The Reptiles Fever—an exotic pet trade expo and was the fourth largest importer of live reptiles in 2016 the largest in the Kansai area, with about 40 trading stalls, &RPWUDGH ,QWKDW\HDU-DSDQLPSRUWHG was also surveyed. All reptile species were recorded to live reptiles and exported 8,702 live reptiles (Ministry of species or subspecies level where possible, as well as )LQDQFH 9LVLWRUVWRUHSWLOHH[SRVKDYHLQFUHDVHG information on the number of animals, price, origin, and over time, with over 20,000 people attending the Tokyo VRXUFH FDSWLYHEUHGRUZLOGFDXJKW ZKHUHSRVVLEOH1R Reptiles World 2016 Show, up from 8,343 in 2011 animals were purchased as part of the survey. -

Muscle Biochemistry and Body Shape Explain Ontogenetic Variation of Anti
© 2016. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2016) 219, 1649-1658 doi:10.1242/jeb.130740 RESEARCH ARTICLE Beyond body size: muscle biochemistry and body shape explain ontogenetic variation of anti-predatory behaviour in the lizard Salvator merianae Fábio Cury de Barros1, JoséEduardo de Carvalho2, Augusto Shinya Abe3 and Tiana Kohlsdorf1,* ABSTRACT When facing a predator and after choosing among possible anti- Anti-predatory behaviour evolves under the strong action of natural predatory strategies, animals are likely to adjust the characteristics selection because the success of individuals avoiding predation and intensity of the elected behavioural response according to the essentially defines their fitness. Choice of anti-predatory strategies is perceived level of predation risk (Brown et al., 2006; Greene, 1988; defined by prey characteristics as well as environmental temperature. Martín and López, 2003; Ydenberg and Dill, 1986). Many factors An additional dimension often relegated in this multilevel equation is might influence the choice and intensity of the elected anti- the ontogenetic component. In the tegu Salvator merianae, adults run predatory tactic, such as local conditions [i.e. environmental away from predators at high temperatures but prefer fighting when it is temperature, amount of light/period of day and vegetation cover/ cold, whereas juveniles exhibit the same flight strategy within a wide terrain characteristics (Savino and Stein, 1989; Christensen and thermal range. Here, we integrate physiology and morphology to Persson, 1993; Brodie and Russell, 1999; Shine et al., 2000, 2003; understand ontogenetic variation in the temperature-dependent shift Schulte et al., 2004; Durso and Mullin, 2014)] or type and density of of anti-predatory behaviour in these lizards. -

Class: Amphibia Amphibians Order
CLASS: AMPHIBIA AMPHIBIANS ANNIELLIDAE (Legless Lizards & Allies) CLASS: AMPHIBIA AMPHIBIANS Anniella (Legless Lizards) ORDER: ANURA FROGS AND TOADS ___Silvery Legless Lizard .......................... DS,RI,UR – uD ORDER: ANURA FROGS AND TOADS BUFONIDAE (True Toad Family) BUFONIDAE (True Toad Family) ___Southern Alligator Lizard ............................ RI,DE – fD Bufo (True Toads) Suborder: SERPENTES SNAKES Bufo (True Toads) ___California (Western) Toad.............. AQ,DS,RI,UR – cN ___California (Western) Toad ............. AQ,DS,RI,UR – cN ANNIELLIDAE (Legless Lizards & Allies) Anniella ___Red-spotted Toad ...................................... AQ,DS - cN BOIDAE (Boas & Pythons) ___Red-spotted Toad ...................................... AQ,DS - cN (Legless Lizards) Charina (Rosy & Rubber Boas) ___Silvery Legless Lizard .......................... DS,RI,UR – uD HYLIDAE (Chorus Frog and Treefrog Family) ___Rosy Boa ............................................ DS,CH,RO – fN HYLIDAE (Chorus Frog and Treefrog Family) Pseudacris (Chorus Frogs) Pseudacris (Chorus Frogs) Suborder: SERPENTES SNAKES ___California Chorus Frog ............ AQ,DS,RI,DE,RO – cN COLUBRIDAE (Colubrid Snakes) ___California Chorus Frog ............ AQ,DS,RI,DE,RO – cN ___Pacific Chorus Frog ....................... AQ,DS,RI,DE – cN Arizona (Glossy Snakes) ___Pacific Chorus Frog ........................AQ,DS,RI,DE – cN BOIDAE (Boas & Pythons) ___Glossy Snake ........................................... DS,SA – cN Charina (Rosy & Rubber Boas) RANIDAE (True Frog Family) -

Brochure. Checklist of Amphibians and Reptiles of the Carrizo Plain
The Carrizo Plain National Monument is cooperatively managed by: Bureau of Land Management The Nature Conservancy California Department of Fish and Game Checklist of blunt-nosed leopard lizard For more information contact: Goodwin Education Center 805-475-2131 Seasonally Open December - May Thursday - Sunday 9:00 am to 4:00 pm Amphibians Bureau of Land Management Bakersfield Field Office 3801 Pegasus Drive Bakersfield, CA 93308 661-391-6000 Monday - Friday and Reptiles 7:30 am to 4:00 pm http: //www.ca.blm.gov/bakersfield/carrizoplain.html Literature Cited Carrizo Plain National Monument Holland, R.F. 1986. Preliminary descriptions of terrestrial natural communities of California. State of California, The Resources Agency, California Department of Fish and Game. 156 Pages. San Luis Obispo County, California Laudenslayer, W.F., Jr. and W.E. Grenfell, Jr. 1983. A list of amphibians, reptiles, birds, and mammals of California. Outdoor California 44:5-14. Mayer, K.E., and W.F. Laudenslayer, Jr., editors. 1988. A guide to wildlife habitats of California. State of California, The Resources Agency, California Department of Forestry and Fire Protection. 166 pages Cover art provided by Miriam Morril Habitat Occurrence Amphibians and Reptiles of the Carrizo Plain KEY TO CODE JUN - Juniper MSC-Mixed Scrubland Night Lizards - Family Xantusiidae National Monument ASC-Alkali Desert Scrub AGS-Annual Grassland Desert Night Lizard JUN/MSC/ASC C C - Common in appropriate habitat. Xantusia vigilis U - Uncommon in appropriate habitat. This checklist includes 26 species, which currently occur within Skinks - Family Scincidae R - Rare, observed only a few times. the Carrizo Plain National Monument. -

Fauna of Australia 2A
FAUNA of AUSTRALIA 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA Mark N. Hutchinson & Stephen C. Donnellan 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA This review summarises the current hypotheses of the origin, antiquity and history of the order Squamata, the dominant living reptile group which comprises the lizards, snakes and worm-lizards. The primary concern here is with the broad relationships and origins of the major taxa rather than with local distributional or phylogenetic patterns within Australia. In our review of the phylogenetic hypotheses, where possible we refer principally to data sets that have been analysed by cladistic methods. Analyses based on anatomical morphological data sets are integrated with the results of karyotypic and biochemical data sets. A persistent theme of this chapter is that for most families there are few cladistically analysed morphological data, and karyotypic or biochemical data sets are limited or unavailable. Biogeographic study, especially historical biogeography, cannot proceed unless both phylogenetic data are available for the taxa and geological data are available for the physical environment. Again, the reader will find that geological data are very uncertain regarding the degree and timing of the isolation of the Australian continent from Asia and Antarctica. In most cases, therefore, conclusions should be regarded very cautiously. The number of squamate families in Australia is low. Five of approximately fifteen lizard families and five or six of eleven snake families occur in the region; amphisbaenians are absent. Opinions vary concerning the actual number of families recognised in the Australian fauna, depending on whether the Pygopodidae are regarded as distinct from the Gekkonidae, and whether sea snakes, Hydrophiidae and Laticaudidae, are recognised as separate from the Elapidae. -
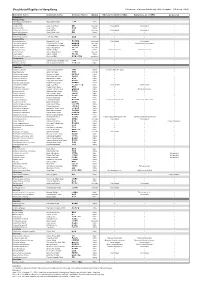
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012)
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012) Scientific name Common name Chinese Name Status Ades & Kendrick (2004) Karsen et al. (1998) Uetz et al. Testudines Platysternidae Platysternon megacephalum Big-headed Terrapin 大頭龜 Native Cheloniidae Caretta caretta Loggerhead Turtle 蠵龜 Uncertain Not included Not included Chelonia mydas Green Turtle 緣海龜 Native Eretmochelys imbricata Hawksbill Turtle 玳瑁 Uncertain Not included Not included Lepidochelys olivacea Pacific Ridley Turtle 麗龜 Native Dermochelyidae Dermochelys coriacea Leatherback Turtle 稜皮龜 Native Geoemydidae Cuora amboinensis Malayan Box Turtle 馬來閉殼龜 Introduced Not included Not included Cuora flavomarginata Yellow-lined Box Terrapin 黃緣閉殼龜 Uncertain Cistoclemmys flavomarginata Cuora trifasciata Three-banded Box Terrapin 三線閉殼龜 Native Mauremys mutica Chinese Pond Turtle 黃喉水龜 Uncertain Mauremys reevesii Reeves' Terrapin 烏龜 Native Chinemys reevesii Chinemys reevesii Ocadia sinensis Chinese Striped Turtle 中華花龜 Uncertain Sacalia bealei Beale's Terrapin 眼斑水龜 Native Trachemys scripta elegans Red-eared Slider 巴西龜 / 紅耳龜 Introduced Trionychidae Palea steindachneri Wattle-necked Soft-shelled Turtle 山瑞鱉 Uncertain Pelodiscus sinensis Chinese Soft-shelled Turtle 中華鱉 / 水魚 Native Squamata - Serpentes Colubridae Achalinus rufescens Rufous Burrowing Snake 棕脊蛇 Native Achalinus refescens (typo) Ahaetulla prasina Jade Vine Snake 綠瘦蛇 Uncertain Amphiesma atemporale Mountain Keelback 無顳鱗游蛇 Native -

Kumejima Island, Japan
O . kikuzatoi through a series of field surveys as below. Current Herpetology 23(2): 73-80, December 2004 (c)2004 by The Herpetological Society of Japan Field Observations on a Highly Endangered Snake, Opisthotropis kikuzatoi (Squamata: Colubridae), Endemic to Kumejima Island, Japan HIDETOSHI OTA* Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-0213, JAPAN Abstract: The Kikuzato's brook snake, Opisthotropis kikuzatoi, is a highly endangered aquatic or semiaquatic species endemic to Kumejima Island of the Okinawa Group, Ryukyu Archipelago. Field studies were carried out for some ecological aspects of this species by visiting its habitat almost every month from April 1996 to March 1997. The results demonstrate that the snake is active almost throughout the year. It is also suggested that the snake tends to be diurnal in the warmer season, and nocturnal in the cooler season. Observations on a case of autonomous emergence onto the land, very slow growth, and predation on small crabs, are also provided. Key words: Opisthotropis kikuzatoi, Field census; Activity pattern; Body temper- ature; Kumejima Island INTRODUCTION broadleaf evergreen forest: see below), led Environment Agency of Japan assign O. The Kikuzato's brook snake Opisthotropis kikuzatoi to the highest Red List Category kikuzatoi, is a small colubrid species endemic (IA) as one of the two most critically endan- to Kumejima Island of the Okinawa Group, gered reptiles of Japan (Matsui, 1991; Ota, Ryukyu Archipelago. Since its original 2000). This snake is also protected by laws of description by Okada and Takara (1958: as a both the National Government of Japan and member of the genus Liopeltis), no more than the Prefectural Government of Okinawa (Ota, ten individuals have been known to science 2000). -

Reptile Diversity in an Amazing Tropical Environment: the West Indies - L
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT - Vol. VIII - Reptile Diversity In An Amazing Tropical Environment: The West Indies - L. Rodriguez Schettino REPTILE DIVERSITY IN AN AMAZING TROPICAL ENVIRONMENT: THE WEST INDIES L. Rodriguez Schettino Department of Zoology, Institute of Ecology and Systematics, Cuba To the memory of Ernest E. Williams and Austin Stanley Rand Keywords: Reptiles, West Indies, geographic distribution, morphological and ecological diversity, ecomorphology, threatens, conservation, Cuba Contents 1. Introduction 2. Reptile diversity 2.1. Morphology 2.2.Habitat 3. West Indian reptiles 3.1. Greater Antilles 3.2. Lesser Antilles 3.3. Bahamas 3.4. Cuba (as a study case) 3.4.1. The Species 3.4.2. Geographic and Ecological Distribution 3.4.3. Ecomorphology 3.4.4. Threats and Conservation 4. Conclusions Acknowledgments Glossary Bibliography Biographical Sketch Summary The main features that differentiate “reptiles” from amphibians are their dry scaled tegument andUNESCO their shelled amniotic eggs. In– modern EOLSS studies, birds are classified under the higher category named “Reptilia”, but the term “reptiles” used here does not include birds. One can externally identify at least, three groups of reptiles: turtles, crocodiles, and lizards and snakes. However, all of these three groups are made up by many species that are differentSAMPLE in some morphological characters CHAPTERS like number of scales, color, size, presence or absence of limbs. Also, the habitat use is quite variable; there are reptiles living in almost all the habitats of the Earth, but the majority of the species are only found in the tropical regions of the world. The West Indies is a region of special interest because of its tropical climate, the high number of species living on the islands, the high level of endemism, the high population densities of many species, and the recognized adaptive radiation that has occurred there in some genera, such as Anolis, Sphaerodactylus, and Tropidophis.