Kumejima Island, Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Species of the Genus Opisthotropis Günther, 1872 from Northern Laos (Squamata: Natricidae)
Zootaxa 3774 (2): 165–182 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3774.2.4 http://zoobank.org/urn:lsid:zoobank.org:pub:933179AB-E8DB-4785-9F79-E0D051E1E398 A new species of the genus Opisthotropis Günther, 1872 from northern Laos (Squamata: Natricidae) ALEXANDRE TEYNIÉ1, ANNE LOTTIER1, PATRICK DAVID*2, TRUONG QUANG NGUYEN3 & GERNOT VOGEL4 1 Société d'Histoire Naturelle Alcide d’Orbigny, 57 rue de Gergovie, F-63170 Aubière, France. E-mail: [email protected] 2 Reptiles & Amphibiens, UMR 7205 OSEB, Département Systématique et Évolution, CP 30, Muséum National d’Histoire Naturelle, 57 rue Cuvier, 75231 Paris Cedex 05, France. E-mail: [email protected] 3 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam Current address: Department of Terrestrial Ecology, Zoological Institute, University of Cologne, Zülpicher Strasse 47b, D-50674 Cologne, Germany. E-mail: [email protected] 4 Society for Southeast Asian Herpetology, Im Sand 3, D-69115 Heidelberg, Germany. E-mail: [email protected] * corresponding author Abstract Two specimens, a male and a female, of the genus Opisthotropis Günther, 1872 were collected in a karst formation of northern Louangphabang (or Luang Prabang) Province, North Laos. These specimens are assigned to the genus Opisthotropis on the basis of their morphology, dentition and cephalic scalation. However, they differ from -

Nansei Islands Biological Diversity Evaluation Project Report 1 Chapter 1
Introduction WWF Japan’s involvement with the Nansei Islands can be traced back to a request in 1982 by Prince Phillip, Duke of Edinburgh. The “World Conservation Strategy”, which was drafted at the time through a collaborative effort by the WWF’s network, the International Union for Conservation of Nature (IUCN), and the United Nations Environment Programme (UNEP), posed the notion that the problems affecting environments were problems that had global implications. Furthermore, the findings presented offered information on precious environments extant throughout the globe and where they were distributed, thereby providing an impetus for people to think about issues relevant to humankind’s harmonious existence with the rest of nature. One of the precious natural environments for Japan given in the “World Conservation Strategy” was the Nansei Islands. The Duke of Edinburgh, who was the President of the WWF at the time (now President Emeritus), naturally sought to promote acts of conservation by those who could see them through most effectively, i.e. pertinent conservation parties in the area, a mandate which naturally fell on the shoulders of WWF Japan with regard to nature conservation activities concerning the Nansei Islands. This marked the beginning of the Nansei Islands initiative of WWF Japan, and ever since, WWF Japan has not only consistently performed globally-relevant environmental studies of particular areas within the Nansei Islands during the 1980’s and 1990’s, but has put pressure on the national and local governments to use the findings of those studies in public policy. Unfortunately, like many other places throughout the world, the deterioration of the natural environments in the Nansei Islands has yet to stop. -

NHBSS 061 1G Hikida Fieldg
Book Review N$7+IST. BULL. S,$0 SOC. 61(1): 41–51, 2015 A Field Guide to the Reptiles of Thailand by Tanya Chan-ard, John W. K. Parr and Jarujin Nabhitabhata. Oxford University Press, New York, 2015. 344 pp. paper. ISBN: 9780199736492. 7KDLUHSWLOHVZHUHÀUVWH[WHQVLYHO\VWXGLHGE\WZRJUHDWKHUSHWRORJLVWV0DOFROP$UWKXU 6PLWKDQG(GZDUG+DUULVRQ7D\ORU7KHLUFRQWULEXWLRQVZHUHSXEOLVKHGDV6MITH (1931, 1935, 1943) and TAYLOR 5HFHQWO\RWKHUERRNVDERXWUHSWLOHVDQGDPSKLELDQV LQ7KDLODQGZHUHSXEOLVKHG HJ&HAN-ARD ET AL., 1999: COX ET AL DVZHOODVPDQ\ SDSHUV+RZHYHUWKHVHERRNVZHUHWD[RQRPLFVWXGLHVDQGQRWJXLGHVIRURUGLQDU\SHRSOH7ZR DGGLWLRQDOÀHOGJXLGHERRNVRQUHSWLOHVRUDPSKLELDQVDQGUHSWLOHVKDYHDOVREHHQSXEOLVKHG 0ANTHEY & GROSSMANN, 1997; DAS EXWWKHVHERRNVFRYHURQO\DSDUWRIWKHIDXQD The book under review is very well prepared and will help us know Thai reptiles better. 2QHRIWKHDXWKRUV-DUXMLQ1DEKLWDEKDWDZDVP\ROGIULHQGIRUPHUO\WKH'LUHFWRURI1DWXUDO +LVWRU\0XVHXPWKH1DWLRQDO6FLHQFH0XVHXP7KDLODQG+HZDVDQH[FHOOHQWQDWXUDOLVW DQGKDGH[WHQVLYHNQRZOHGJHDERXW7KDLDQLPDOVHVSHFLDOO\DPSKLELDQVDQGUHSWLOHV,Q ZHYLVLWHG.KDR6RL'DR:LOGOLIH6DQFWXDU\WRVXUYH\KHUSHWRIDXQD+HDGYLVHGXV WRGLJTXLFNO\DURXQGWKHUH:HFROOHFWHGIRXUVSHFLPHQVRIDibamusZKLFKZHGHVFULEHG DVDQHZVSHFLHVDibamus somsaki +ONDA ET AL 1RZ,DPYHU\JODGWRNQRZWKDW WKLVERRNZDVSXEOLVKHGE\KLPDQGKLVFROOHDJXHV8QIRUWXQDWHO\KHSDVVHGDZD\LQ +LVXQWLPHO\GHDWKPD\KDYHGHOD\HGWKHSXEOLFDWLRQRIWKLVERRN7KHERRNLQFOXGHVQHDUO\ DOOQDWLYHUHSWLOHV PRUHWKDQVSHFLHV LQ7KDLODQGDQGPRVWSLFWXUHVZHUHGUDZQZLWK H[FHOOHQWGHWDLO,WLVDYHU\JRRGÀHOGJXLGHIRULGHQWLÀFDWLRQRI7KDLUHSWLOHVIRUVWXGHQWV -

Expanded Description of a Chinese Endemic Snake Opisthotropis Cheni (Serpentes: Colubridae: Natricinae)
Asian Herpetological Research 2010, 1(1): 57-60 DOI: 10.3724/SP.J.1245.2010.00057 Expanded Description of a Chinese Endemic Snake Opisthotropis cheni (Serpentes: Colubridae: Natricinae) LI Cao1, LIU Qin1 and GUO Peng 1, 2* 1 Department of Life Sciences and Food Engineering, Yibin University, Yibin 644000, Sichuan, China 2 Chendgu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China Abstract Based on seven newly-collected specimens, we provide an expanded description for the rare Chinese snake Opisthotropis cheni. The new specimens are consistent with the type series in scale counts and body dimensions. How- ever, two individuals lack yellow cross-bands that are apparent in the type specimens. A key to the ten Chinese species of Opisthotropis is provided. Keywords snake, Opisthotropis cheni, morphology, China 1. Introduction 070140, 071041, 071046-071050) (Table 1), collected from the Nanling National Nature Reserve in Ruyuan The genus Opisthotropis Gǘnther (1872) is comprised of County, Guangdong Province, China (Figure 1), were 17 currently recognized species, and is widely distributed morphologically examined in this work. The specimens in eastern, southern, and southeastern Asia (Uetz, 2010). were preserved in 8% formalin for initial fixation, and Members of this genus are all small aquatic snakes, later transferred to 75% ethanol. All specimens were de- mainly inhabiting rapidly flowing streams or small rivers. posited at Yibin University (YBU), Sichuan Province, Ten species of Opisthotropis occur in China (Zhao, China. 2006). Snout-vent length (SVL) and tail length (TL) were Zhao (1999) described Opisthotropis cheni based on measured using a meter ruler to the nearest millimeter, four specimens collected from Mt. -

Antimicrobial Peptides in Reptiles
Pharmaceuticals 2014, 7, 723-753; doi:10.3390/ph7060723 OPEN ACCESS pharmaceuticals ISSN 1424-8247 www.mdpi.com/journal/pharmaceuticals Review Antimicrobial Peptides in Reptiles Monique L. van Hoek National Center for Biodefense and Infectious Diseases, and School of Systems Biology, George Mason University, MS1H8, 10910 University Blvd, Manassas, VA 20110, USA; E-Mail: [email protected]; Tel.: +1-703-993-4273; Fax: +1-703-993-7019. Received: 6 March 2014; in revised form: 9 May 2014 / Accepted: 12 May 2014 / Published: 10 June 2014 Abstract: Reptiles are among the oldest known amniotes and are highly diverse in their morphology and ecological niches. These animals have an evolutionarily ancient innate-immune system that is of great interest to scientists trying to identify new and useful antimicrobial peptides. Significant work in the last decade in the fields of biochemistry, proteomics and genomics has begun to reveal the complexity of reptilian antimicrobial peptides. Here, the current knowledge about antimicrobial peptides in reptiles is reviewed, with specific examples in each of the four orders: Testudines (turtles and tortosises), Sphenodontia (tuataras), Squamata (snakes and lizards), and Crocodilia (crocodilans). Examples are presented of the major classes of antimicrobial peptides expressed by reptiles including defensins, cathelicidins, liver-expressed peptides (hepcidin and LEAP-2), lysozyme, crotamine, and others. Some of these peptides have been identified and tested for their antibacterial or antiviral activity; others are only predicted as possible genes from genomic sequencing. Bioinformatic analysis of the reptile genomes is presented, revealing many predicted candidate antimicrobial peptides genes across this diverse class. The study of how these ancient creatures use antimicrobial peptides within their innate immune systems may reveal new understandings of our mammalian innate immune system and may also provide new and powerful antimicrobial peptides as scaffolds for potential therapeutic development. -

Venom Proteomics and Antivenom Neutralization for the Chinese
www.nature.com/scientificreports OPEN Venom proteomics and antivenom neutralization for the Chinese eastern Russell’s viper, Daboia Received: 27 September 2017 Accepted: 6 April 2018 siamensis from Guangxi and Taiwan Published: xx xx xxxx Kae Yi Tan1, Nget Hong Tan1 & Choo Hock Tan2 The eastern Russell’s viper (Daboia siamensis) causes primarily hemotoxic envenomation. Applying shotgun proteomic approach, the present study unveiled the protein complexity and geographical variation of eastern D. siamensis venoms originated from Guangxi and Taiwan. The snake venoms from the two geographical locales shared comparable expression of major proteins notwithstanding variability in their toxin proteoforms. More than 90% of total venom proteins belong to the toxin families of Kunitz-type serine protease inhibitor, phospholipase A2, C-type lectin/lectin-like protein, serine protease and metalloproteinase. Daboia siamensis Monovalent Antivenom produced in Taiwan (DsMAV-Taiwan) was immunoreactive toward the Guangxi D. siamensis venom, and efectively neutralized the venom lethality at a potency of 1.41 mg venom per ml antivenom. This was corroborated by the antivenom efective neutralization against the venom procoagulant (ED = 0.044 ± 0.002 µl, 2.03 ± 0.12 mg/ml) and hemorrhagic (ED50 = 0.871 ± 0.159 µl, 7.85 ± 3.70 mg/ ml) efects. The hetero-specifc Chinese pit viper antivenoms i.e. Deinagkistrodon acutus Monovalent Antivenom and Gloydius brevicaudus Monovalent Antivenom showed negligible immunoreactivity and poor neutralization against the Guangxi D. siamensis venom. The fndings suggest the need for improving treatment of D. siamensis envenomation in the region through the production and the use of appropriate antivenom. Daboia is a genus of the Viperinae subfamily (family: Viperidae), comprising a group of vipers commonly known as Russell’s viper native to the Old World1. -
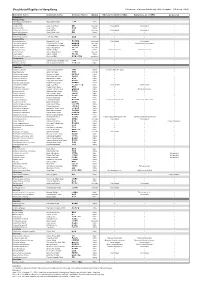
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012)
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012) Scientific name Common name Chinese Name Status Ades & Kendrick (2004) Karsen et al. (1998) Uetz et al. Testudines Platysternidae Platysternon megacephalum Big-headed Terrapin 大頭龜 Native Cheloniidae Caretta caretta Loggerhead Turtle 蠵龜 Uncertain Not included Not included Chelonia mydas Green Turtle 緣海龜 Native Eretmochelys imbricata Hawksbill Turtle 玳瑁 Uncertain Not included Not included Lepidochelys olivacea Pacific Ridley Turtle 麗龜 Native Dermochelyidae Dermochelys coriacea Leatherback Turtle 稜皮龜 Native Geoemydidae Cuora amboinensis Malayan Box Turtle 馬來閉殼龜 Introduced Not included Not included Cuora flavomarginata Yellow-lined Box Terrapin 黃緣閉殼龜 Uncertain Cistoclemmys flavomarginata Cuora trifasciata Three-banded Box Terrapin 三線閉殼龜 Native Mauremys mutica Chinese Pond Turtle 黃喉水龜 Uncertain Mauremys reevesii Reeves' Terrapin 烏龜 Native Chinemys reevesii Chinemys reevesii Ocadia sinensis Chinese Striped Turtle 中華花龜 Uncertain Sacalia bealei Beale's Terrapin 眼斑水龜 Native Trachemys scripta elegans Red-eared Slider 巴西龜 / 紅耳龜 Introduced Trionychidae Palea steindachneri Wattle-necked Soft-shelled Turtle 山瑞鱉 Uncertain Pelodiscus sinensis Chinese Soft-shelled Turtle 中華鱉 / 水魚 Native Squamata - Serpentes Colubridae Achalinus rufescens Rufous Burrowing Snake 棕脊蛇 Native Achalinus refescens (typo) Ahaetulla prasina Jade Vine Snake 綠瘦蛇 Uncertain Amphiesma atemporale Mountain Keelback 無顳鱗游蛇 Native -

List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Cheloniidae 4 Loggerhead Turtle Caretta caretta Green Turtle Chelonia mydas Hawksbill Turtle Eretmochelys imbricata Olive Ridley Turtle Lepidochelys olivacea Dermochelyidae 1 Leatherback Turtle Dermochelys coriacea Emydidae 1 Red-eared Slider * Trachemys scripta elegans Geoemydidae 3 Three-banded Box Turtle Cuora trifasciata Reeves' Turtle Mauremys reevesii Beale's Turtle Sacalia bealei Platysternidae 1 Big-headed Turtle Platysternon megacephalum Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Agamidae 1 Changeable Lizard Calotes versicolor Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Long-tailed Skink Eutropis longicaudata Chinese Skink Plestiodon chinensis chinensis Five-striped Blue-tailed Skink Plestiodon elegans Blue-tailed Skink Plestiodon quadrilineatus Vietnamese Five-lined Skink Plestiodon tamdaoensis Slender Forest Skink Scincella modesta Reeve's Smooth Skink Scincella reevesii Brown Forest Skink Sphenomorphus incognitus Indian Forest Skink Sphenomorphus indicus Chinese Waterside Skink Tropidophorus sinicus Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek's Burrowing Lizard Dibamus bogadeki Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko Gekko gecko Bowring's Gecko Hemidactylus bowringii Brook's Gecko* Hemidactylus brookii House Gecko* Hemidactylus -
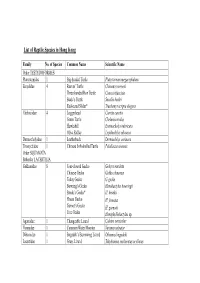
List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Platysternidae 1 Big-headed Turtle Platysternum megacephalum Emydidae 4 Reeves’ Turtle Chinemys reevesii Three-banded Box Turtle Cuora trifasciata Beale’s Turtle Sacalia bealei Red-eared Slider* Trachemys scripta elegans Cheloniidae 4 Loggerhead Caretta caretta Green Turtle Chelonia mydas Hawksbill Eretmochelys imbricata Olive Ridley Lepidochelys olivacea Dermochelyidae 1 Leatherback Dermochelys coriacea Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko G. gecko Bowring’s Gecko Hemidactylus bowringii Brook’s Gecko* H. brookii House Gecko H. frenatus Garnot’s Gecko H. garnotii Tree Gecko Hemiphyllodactylus sp. Agamidae 1 Changeable Lizard Calotes versicolor Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek’s Burrowing Lizard Dibamus bogadeki Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Chinese Skink Eumeces chinensis chinensis Five-striped Blue-tailed Skink E. elegans Blue-tailed Skink E. quadrilineatus Vietnamese Five-lined Skink E. tamdaoensis Long-tailed Skink Mabuya longicaudata Slender Forest Skink Scincella modesta Reeve’s Smooth Skink S. reevesii Indian Forest Skink Sphenomorphus indicus Brown Forest Skink S. incognitus Chinese Waterside Skink Tropidophorus sinicus Order SQUAMATA Suborder SERPENTES Typhlopidae 3 Lazell’s Blind Snake Typhlops lazelli White-headed Blind Snake Ramphotyphlops albiceps Common Blind Snake R. braminus Boidae 1 Burmese Python Python molurus bivittatus Colubridae 34 Rufous Burrowing Snake Achalinus rufescens Jade Vine Snake Ahaetulla prasina medioxima Mountain Keelback Amphiesma atemporale White-browed Keelback A. boulengeri Buff-striped Keelback A. -

Wallach Et Al., 2009 and Kaiser Et Al., 2013)
SNAKES of the WORLD A Catalogue of Living and Extinct Species Van Wallach Kenneth L. Williams Jeff Boundy K21592.indb 3 4/16/14 3:24 PM CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2014 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed on acid-free paper Version Date: 20140108 International Standard Book Number-13: 978-1-4822-0847-4 (Hardback) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information storage or retrieval system, without written permission from the publishers. For permission to photocopy or use material electronically from this work, please access www.copyright.com (http://www.copyright.com/) or contact the Copyright Clearance Center, Inc. -

Bulletin 110 FINAL EDIT.Indd
The HERPETOLOGICAL BULLETIN Number 110 – Winter 2009 PUBLISHED BY THE BRITISH HERPETOLOGICAL SOCIETY THE HERPETOLOGICAL BULLETIN Contents NEWS REPO R TS . 1 RESEA R CH ABST R ACTS . 5 RESEA R CH AR TICLES Grassland snake assemblages in central and western Pennsylvania and northeastern Ohio, USA Walter E. Meshaka, Jr., Samuel D. Marshall, Timothy J. Guiher and Lindsay Zemba . 8 Recent data on the distribution of lizards and snakes of the Seychelles Sara Rocha, D. James Harris, Ana Perera, Andreia Silva, Raquel Vasconcelos and Miguel A. Carretero . 20 An undescribed gecko (Gekkonidae: Cyrtodactylus) from Deer Cave, Gunung Mulu National Park, Sarawak, with comments on the distribution of Bornean cave geckos Donald A. McFarlane, Joyce Lundberg and Keith Christenson . 33 Conspicuous tail coloration in Vipera berus Kevin Palmer................................................... 36 CAPTIVE HUSBA N D R Y Notes on the captive husbandry and breeding of the Shovel-footed Squeaker, Arthroleptis stenodactylus (Pfeffer 1893) Benjamin Tapley . 38 BOOK REVIEW Wildlife Monographs: Living Dinosaurs & Other Reptiles by Heather Angel Kate Statham...................................................- Registered Charity No. 205666 - 42 ACK N OWLEDGEME N TS . 44 - Registered Charity No. 205666 - THE HERPETOLOGICAL BULLETIN The Herpetological Bulletin is produced quarterly and publishes, in English, a range of articles concerned with herpetology. These include society news, selected news reports, full-length papers of a semi- technical nature, new methodologies, natural history notes, book reviews, letters from readers and other items of general herpetological interest. Emphasis is placed on natural history, conservation, captive breeding and husbandry, veterinary and behavioural aspects. Articles reporting the results of experimental research, descriptions of new taxa, or taxonomic revisions should be submitted to The Herpetological Journal (see inside back cover for Editor’s address). -

On the Generic Taxonomy of Opisthotropis Balteata (Cope, 1895) (Squamata: Colubridae: Natricinae): Taxonomic Revision of Two Natricine Genera
Asian Herpetological Research 2019, 10(2): 105–128 ORIGINAL ARTICLE DOI: 10.16373/j.cnki.ahr.180091 On the Generic Taxonomy of Opisthotropis balteata (Cope, 1895) (Squamata: Colubridae: Natricinae): Taxonomic Revision of Two Natricine Genera Jinlong REN1,2,3, Kai WANG4, Peng GUO5, Yingyong WANG6, Tao Thien NGUYEN7,8 and Jiatang LI1,2,9* 1 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization and Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan 610041, China 2 Center for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, Kunming, Yunnan 650223, China 3 University of Chinese Academy of Sciences, Beijing 100049, China 4 Sam Noble Oklahoma Museum of Natural History and Department of Biology, University of Oklahoma, Norman, Oklahoma 73019, USA 5 College of Life Sciences and Food Engineering, Yibin University, Yibin, Sichuan 644007, China 6 State Key Laboratory of Biocontrol / The Museum of Biology, School of Life Sciences, Sun Yat-sen University, Guangzhou, Guangdong 510275, China 7 Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam 8 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam 9 Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin, Nay Pyi Taw 05282, Myanmar Abstract The single prefrontal configuration has historically been used as an important diagnostic character for many natricine taxa. For example, the genus Trimerodytes Cope, 1895 was long been regarded as a junior synonym of Opisthotropis Günther, 1872 for their similar prefrontal configurations and the type species, T.