Bulletin 110 FINAL EDIT.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution and Habitat of the Invasive Giant Day Gecko Phelsuma Grandis Gray 1870
Phelsuma 22 (2014); 13-28 Distribution and habitat of the invasive giant day gecko Phelsuma grandis Gray 1870 (Sauria: Gekkonidae) in Reunion Island, and conservation implication Mickaël Sanchez1,* and Jean-Michel Probst1,2 1 Nature Océan Indien, 6, Lotissement les Magnolias, Rivière des Roches, 97470 Saint-Benoît, La Réunion, France 2 Nature et Patrimoine, 2, Allée Mangaron, Dos d’Ane, 97419 La Possession, La Réunion, France * Corresponding author; e-mail: [email protected] Abstract. The giant day gecko Phelsuma grandis, endemic to Madagascar, was introduced to Reunion Island (Indian Ocean) in the mid-1990s. No studies have been conducted so far to define its precise distribution and habitat. To fill the knowledge gap about this invasive species, we compiled available data and performed field work during 2007-2014. We detected 13 distinct populations of P. grandis, occurring mainly in the northern part of Reunion Island and on the west coast. This gecko inhabits human disturbed areas (gardens, urban parks, bamboos, orchards, coconuts, and banana plantation) and secondary habitats (shrubby savanna, and secondary dry woodlands, secondary dry and wet thickets). Its distribution strongly suggests that saltatory dispersal (through deliberate and/or accidental transport) and natural colonization are the mechanisms of spreading through Reunion Island. All our data in combination with both P. grandis ecology and native environmental range suggested that this gecko may colonize native forest, and constitutes a potential important threat to the native biodiversity of Reunion Island (arthropods and lizards). Key words. Phelsuma grandis, Reunion Island, distribution, invasive species, conservation. Introduction Day geckos of the genus Phelsuma are distributed in the western Indian Ocean (Austin et al. -

A New Species of the Genus Opisthotropis Günther, 1872 from Northern Laos (Squamata: Natricidae)
Zootaxa 3774 (2): 165–182 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3774.2.4 http://zoobank.org/urn:lsid:zoobank.org:pub:933179AB-E8DB-4785-9F79-E0D051E1E398 A new species of the genus Opisthotropis Günther, 1872 from northern Laos (Squamata: Natricidae) ALEXANDRE TEYNIÉ1, ANNE LOTTIER1, PATRICK DAVID*2, TRUONG QUANG NGUYEN3 & GERNOT VOGEL4 1 Société d'Histoire Naturelle Alcide d’Orbigny, 57 rue de Gergovie, F-63170 Aubière, France. E-mail: [email protected] 2 Reptiles & Amphibiens, UMR 7205 OSEB, Département Systématique et Évolution, CP 30, Muséum National d’Histoire Naturelle, 57 rue Cuvier, 75231 Paris Cedex 05, France. E-mail: [email protected] 3 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam Current address: Department of Terrestrial Ecology, Zoological Institute, University of Cologne, Zülpicher Strasse 47b, D-50674 Cologne, Germany. E-mail: [email protected] 4 Society for Southeast Asian Herpetology, Im Sand 3, D-69115 Heidelberg, Germany. E-mail: [email protected] * corresponding author Abstract Two specimens, a male and a female, of the genus Opisthotropis Günther, 1872 were collected in a karst formation of northern Louangphabang (or Luang Prabang) Province, North Laos. These specimens are assigned to the genus Opisthotropis on the basis of their morphology, dentition and cephalic scalation. However, they differ from -

NHBSS 061 1G Hikida Fieldg
Book Review N$7+IST. BULL. S,$0 SOC. 61(1): 41–51, 2015 A Field Guide to the Reptiles of Thailand by Tanya Chan-ard, John W. K. Parr and Jarujin Nabhitabhata. Oxford University Press, New York, 2015. 344 pp. paper. ISBN: 9780199736492. 7KDLUHSWLOHVZHUHÀUVWH[WHQVLYHO\VWXGLHGE\WZRJUHDWKHUSHWRORJLVWV0DOFROP$UWKXU 6PLWKDQG(GZDUG+DUULVRQ7D\ORU7KHLUFRQWULEXWLRQVZHUHSXEOLVKHGDV6MITH (1931, 1935, 1943) and TAYLOR 5HFHQWO\RWKHUERRNVDERXWUHSWLOHVDQGDPSKLELDQV LQ7KDLODQGZHUHSXEOLVKHG HJ&HAN-ARD ET AL., 1999: COX ET AL DVZHOODVPDQ\ SDSHUV+RZHYHUWKHVHERRNVZHUHWD[RQRPLFVWXGLHVDQGQRWJXLGHVIRURUGLQDU\SHRSOH7ZR DGGLWLRQDOÀHOGJXLGHERRNVRQUHSWLOHVRUDPSKLELDQVDQGUHSWLOHVKDYHDOVREHHQSXEOLVKHG 0ANTHEY & GROSSMANN, 1997; DAS EXWWKHVHERRNVFRYHURQO\DSDUWRIWKHIDXQD The book under review is very well prepared and will help us know Thai reptiles better. 2QHRIWKHDXWKRUV-DUXMLQ1DEKLWDEKDWDZDVP\ROGIULHQGIRUPHUO\WKH'LUHFWRURI1DWXUDO +LVWRU\0XVHXPWKH1DWLRQDO6FLHQFH0XVHXP7KDLODQG+HZDVDQH[FHOOHQWQDWXUDOLVW DQGKDGH[WHQVLYHNQRZOHGJHDERXW7KDLDQLPDOVHVSHFLDOO\DPSKLELDQVDQGUHSWLOHV,Q ZHYLVLWHG.KDR6RL'DR:LOGOLIH6DQFWXDU\WRVXUYH\KHUSHWRIDXQD+HDGYLVHGXV WRGLJTXLFNO\DURXQGWKHUH:HFROOHFWHGIRXUVSHFLPHQVRIDibamusZKLFKZHGHVFULEHG DVDQHZVSHFLHVDibamus somsaki +ONDA ET AL 1RZ,DPYHU\JODGWRNQRZWKDW WKLVERRNZDVSXEOLVKHGE\KLPDQGKLVFROOHDJXHV8QIRUWXQDWHO\KHSDVVHGDZD\LQ +LVXQWLPHO\GHDWKPD\KDYHGHOD\HGWKHSXEOLFDWLRQRIWKLVERRN7KHERRNLQFOXGHVQHDUO\ DOOQDWLYHUHSWLOHV PRUHWKDQVSHFLHV LQ7KDLODQGDQGPRVWSLFWXUHVZHUHGUDZQZLWK H[FHOOHQWGHWDLO,WLVDYHU\JRRGÀHOGJXLGHIRULGHQWLÀFDWLRQRI7KDLUHSWLOHVIRUVWXGHQWV -

Expanded Description of a Chinese Endemic Snake Opisthotropis Cheni (Serpentes: Colubridae: Natricinae)
Asian Herpetological Research 2010, 1(1): 57-60 DOI: 10.3724/SP.J.1245.2010.00057 Expanded Description of a Chinese Endemic Snake Opisthotropis cheni (Serpentes: Colubridae: Natricinae) LI Cao1, LIU Qin1 and GUO Peng 1, 2* 1 Department of Life Sciences and Food Engineering, Yibin University, Yibin 644000, Sichuan, China 2 Chendgu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China Abstract Based on seven newly-collected specimens, we provide an expanded description for the rare Chinese snake Opisthotropis cheni. The new specimens are consistent with the type series in scale counts and body dimensions. How- ever, two individuals lack yellow cross-bands that are apparent in the type specimens. A key to the ten Chinese species of Opisthotropis is provided. Keywords snake, Opisthotropis cheni, morphology, China 1. Introduction 070140, 071041, 071046-071050) (Table 1), collected from the Nanling National Nature Reserve in Ruyuan The genus Opisthotropis Gǘnther (1872) is comprised of County, Guangdong Province, China (Figure 1), were 17 currently recognized species, and is widely distributed morphologically examined in this work. The specimens in eastern, southern, and southeastern Asia (Uetz, 2010). were preserved in 8% formalin for initial fixation, and Members of this genus are all small aquatic snakes, later transferred to 75% ethanol. All specimens were de- mainly inhabiting rapidly flowing streams or small rivers. posited at Yibin University (YBU), Sichuan Province, Ten species of Opisthotropis occur in China (Zhao, China. 2006). Snout-vent length (SVL) and tail length (TL) were Zhao (1999) described Opisthotropis cheni based on measured using a meter ruler to the nearest millimeter, four specimens collected from Mt. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

9. Les Reptiles
9. Les reptiles 256 9. Les reptiles Olivier S.G. Pauwels Etat des recherches du site Ramsar du bas Ogooué n’a pas fait La liste complète des reptiles recensés 9.1. Jeune crocodile à long museau prenant le soleil l’objet de recherches systématiques et qu’il à ce jour des deux zones est donnée dans sur une branche surplombant la lagune Nkomi herpétologiques reste encore certainement des espèces à le Tableau en annexe. Elle reprend les (Photo : Gaël R. Vande weghe). La première tentative de liste des espèces mettre en évidence, la synthèse des données mentions de Pauwels (1995) pour les serpents de reptiles du Gabon, publiée en 1900 par éparses accumulées depuis plus d’un siècle aquatiques du genre Grayia, la synthèse sur 9.2. Pelusios niger adulte dans le village de le zoologiste belge Georges A. Boulenger, permet néanmoins d’avoir une bonne idée de les reptiles gabonais produite par Pauwels & Tsam Tsam sur le lac Oguemoué. Cette tortue est incluait notamment un certain nombre ce qui y vit. Vande weghe (2008) et certains travaux plus consommée quand elle se retrouve piégée dans les de spécimens capturés dans la zone de récents fournissant des données sur cette zone filets de pêche (Photo et commentaire : Heather Lambaréné par l’exploratrice anglaise Mary (Bell & Stuart, sans date ; Pauwels et al., Arrowood). Kingsley pendant son expédition en 1895, Diversité et richesse 2011, 2016, 2017 ; Pauwels & Vande weghe, et par le missionnaire protestant français 2011 ; Pauwels, 2016 ; ces dernières incluant Ernest Haug qui était posté à Ngomo. Au spécifique les observations de terrain occasionnelles que sein du site Ramsar, la localité de loin la Nous avons effectué des recherches biblio- nous avons faites personnellement à Port- mieux étudiée herpétologiquement à ce jour graphiques approfondies pour deux zones Gentil, Lambaréné, Ndjolé et environs de est Lambaréné (voir notamment les travaux adjacentes du bas Ogooué, et n’avons pris en 2001 à 2011). -

Parachute Geckos Free Fall Into Synonymy Gekko Phylogeny, And
Molecular Phylogenetics and Evolution 146 (2020) 106731 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Parachute geckos free fall into synonymy: Gekko phylogeny, and a new T subgeneric classifcation, inferred from thousands of ultraconserved elements ⁎ Perry L. Wood Jr.a, , Xianguang Guoa,b, Scott L. Traversa, Yong-Chao Sua,c, Karen V. Olsona, Aaron M. Bauerd, L. Lee Grismere, Cameron D. Silerf, Robert G. Moylea, Michael J. Anderseng, Rafe M. Browna a Biodiversity Institute and Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, KS 66045, USA b Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China c Department of Biomedical Science and Environmental Biology, Kaohsiung Medical University, Kaohsiung City 80708, Taiwan d Department of Biology and Center for Biodiversity and Ecosystem Stewardship , 800 Lancaster Avenue, Villanova University, Villanova, PA 19085, USA e Herpetology Laboratory, Department of Biology, La Sierra University, Riverside, CA 92515, USA f Department of Biology and Sam Noble Oklahoma Museum of Natural History, University of Oklahoma, Norman, OK 73072-7029, USA g Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, NM 87131, USA ARTICLE INFO ABSTRACT Keywords: Recent phylogenetic studies of gekkonid lizards have revealed unexpected, widespread paraphyly and polyphyly Luperosaurus among genera, unclear generic boundaries, and a tendency towards the nesting of taxa exhibiting specialized, Ptychozoon apomorphic morphologies within geographically widespread “generalist” clades. This is especially true in Phylogenomics Australasia, where monophyly of Gekko proper has been questioned with respect to phenotypically ornate fap- Species tree legged geckos of the genus Luperosaurus, the Philippine false geckos of the genus Pseudogekko, and even the Subgenera elaborately “derived” parachute geckos of the genus Ptychozoon. -

Contents Herpetological Journal
British Herpetological Society Herpetological Journal Volume 31, Number 3, 2021 Contents Full papers Killing them softly: a review on snake translocation and an Australian case study 118-131 Jari Cornelis, Tom Parkin & Philip W. Bateman Potential distribution of the endemic Short-tailed ground agama Calotes minor (Hardwicke & Gray, 132-141 1827) in drylands of the Indian sub-continent Ashish Kumar Jangid, Gandla Chethan Kumar, Chandra Prakash Singh & Monika Böhm Repeated use of high risk nesting areas in the European whip snake, Hierophis viridiflavus 142-150 Xavier Bonnet, Jean-Marie Ballouard, Gopal Billy & Roger Meek The Herpetological Journal is published quarterly by Reproductive characteristics, diet composition and fat reserves of nose-horned vipers (Vipera 151-161 the British Herpetological Society and is issued free to ammodytes) members. Articles are listed in Current Awareness in Marko Anđelković, Sonja Nikolić & Ljiljana Tomović Biological Sciences, Current Contents, Science Citation Index and Zoological Record. Applications to purchase New evidence for distinctiveness of the island-endemic Príncipe giant tree frog (Arthroleptidae: 162-169 copies and/or for details of membership should be made Leptopelis palmatus) to the Hon. Secretary, British Herpetological Society, The Kyle E. Jaynes, Edward A. Myers, Robert C. Drewes & Rayna C. Bell Zoological Society of London, Regent’s Park, London, NW1 4RY, UK. Instructions to authors are printed inside the Description of the tadpole of Cruziohyla calcarifer (Boulenger, 1902) (Amphibia, Anura, 170-176 back cover. All contributions should be addressed to the Phyllomedusidae) Scientific Editor. Andrew R. Gray, Konstantin Taupp, Loic Denès, Franziska Elsner-Gearing & David Bewick A new species of Bent-toed gecko (Squamata: Gekkonidae: Cyrtodactylus Gray, 1827) from the Garo 177-196 Hills, Meghalaya State, north-east India, and discussion of morphological variation for C. -

1 7 an Identification Key to the Geckos of the Seychelles
HERPETOLOGICAL JOURNAL. Vol. I. pp. 17-19 (19X5l 17 AN IDENTIFICATION KEY TO THE GECKOS OF THE SEYCHELLES, WITH BRIEF NOTES ON THEIR DISTRIBUTIONS AND HABITS ANDREW S. GARDNER Department of Zoology, University of Aberdren. Ti/lydrone Avenue, Aberdeen AB9 2TN. U. K. Present addresses: The Calton Laboratory. Department of Genetics and Biomet IT, Universif.I' Co/legr London. Wo/f�·on !-louse, 4 Stephenson Wa r London NWI 21-11'.. U.K. (A ccepted 24. /0. 84) INTRODUCTION 4. Scales on chest and at least anterior of belly keeled. Underside white. Phe!suma astriata The Republic of Seychelles, lying in the western Tornier. 5. Indian Ocean consists of a group of mountainous, granitic islands, and a large number of outlying coral Scales on chest and belly not keeled. 6. atolls and sand cays, distributed over 400,000 km2 of sea. There are over a hundred islands, ranging in size 5. Subcaudal scales keeled and not transversely from Mahe, at 148 km2 to islands little more than enlarged in original tails. Ground colour of emergent rocks. A total of eighteen species of lizard, rump and tail usually bright blue, and of from three families are recorded from the Seychelles nanks, green. Tail unmarked or spotted with (Gardner, 1984). The best represented family is the red. Red transverse neck bars often reduced or Gekkonidae with eleven species, fo ur of which are absent. Phe/suma astriata astriata Tornier i endemic to the islands. The identification key 90 1. presented here should enable interested naturalists to Subcaudal scales unkeeled and transversely identify any gecko encountered in the Seychelles to the enlarged in original tails. -
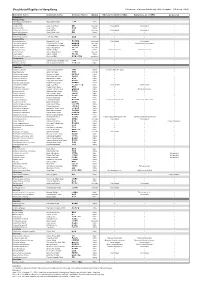
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012)
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012) Scientific name Common name Chinese Name Status Ades & Kendrick (2004) Karsen et al. (1998) Uetz et al. Testudines Platysternidae Platysternon megacephalum Big-headed Terrapin 大頭龜 Native Cheloniidae Caretta caretta Loggerhead Turtle 蠵龜 Uncertain Not included Not included Chelonia mydas Green Turtle 緣海龜 Native Eretmochelys imbricata Hawksbill Turtle 玳瑁 Uncertain Not included Not included Lepidochelys olivacea Pacific Ridley Turtle 麗龜 Native Dermochelyidae Dermochelys coriacea Leatherback Turtle 稜皮龜 Native Geoemydidae Cuora amboinensis Malayan Box Turtle 馬來閉殼龜 Introduced Not included Not included Cuora flavomarginata Yellow-lined Box Terrapin 黃緣閉殼龜 Uncertain Cistoclemmys flavomarginata Cuora trifasciata Three-banded Box Terrapin 三線閉殼龜 Native Mauremys mutica Chinese Pond Turtle 黃喉水龜 Uncertain Mauremys reevesii Reeves' Terrapin 烏龜 Native Chinemys reevesii Chinemys reevesii Ocadia sinensis Chinese Striped Turtle 中華花龜 Uncertain Sacalia bealei Beale's Terrapin 眼斑水龜 Native Trachemys scripta elegans Red-eared Slider 巴西龜 / 紅耳龜 Introduced Trionychidae Palea steindachneri Wattle-necked Soft-shelled Turtle 山瑞鱉 Uncertain Pelodiscus sinensis Chinese Soft-shelled Turtle 中華鱉 / 水魚 Native Squamata - Serpentes Colubridae Achalinus rufescens Rufous Burrowing Snake 棕脊蛇 Native Achalinus refescens (typo) Ahaetulla prasina Jade Vine Snake 綠瘦蛇 Uncertain Amphiesma atemporale Mountain Keelback 無顳鱗游蛇 Native -

Kumejima Island, Japan
O . kikuzatoi through a series of field surveys as below. Current Herpetology 23(2): 73-80, December 2004 (c)2004 by The Herpetological Society of Japan Field Observations on a Highly Endangered Snake, Opisthotropis kikuzatoi (Squamata: Colubridae), Endemic to Kumejima Island, Japan HIDETOSHI OTA* Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-0213, JAPAN Abstract: The Kikuzato's brook snake, Opisthotropis kikuzatoi, is a highly endangered aquatic or semiaquatic species endemic to Kumejima Island of the Okinawa Group, Ryukyu Archipelago. Field studies were carried out for some ecological aspects of this species by visiting its habitat almost every month from April 1996 to March 1997. The results demonstrate that the snake is active almost throughout the year. It is also suggested that the snake tends to be diurnal in the warmer season, and nocturnal in the cooler season. Observations on a case of autonomous emergence onto the land, very slow growth, and predation on small crabs, are also provided. Key words: Opisthotropis kikuzatoi, Field census; Activity pattern; Body temper- ature; Kumejima Island INTRODUCTION broadleaf evergreen forest: see below), led Environment Agency of Japan assign O. The Kikuzato's brook snake Opisthotropis kikuzatoi to the highest Red List Category kikuzatoi, is a small colubrid species endemic (IA) as one of the two most critically endan- to Kumejima Island of the Okinawa Group, gered reptiles of Japan (Matsui, 1991; Ota, Ryukyu Archipelago. Since its original 2000). This snake is also protected by laws of description by Okada and Takara (1958: as a both the National Government of Japan and member of the genus Liopeltis), no more than the Prefectural Government of Okinawa (Ota, ten individuals have been known to science 2000). -

List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Cheloniidae 4 Loggerhead Turtle Caretta caretta Green Turtle Chelonia mydas Hawksbill Turtle Eretmochelys imbricata Olive Ridley Turtle Lepidochelys olivacea Dermochelyidae 1 Leatherback Turtle Dermochelys coriacea Emydidae 1 Red-eared Slider * Trachemys scripta elegans Geoemydidae 3 Three-banded Box Turtle Cuora trifasciata Reeves' Turtle Mauremys reevesii Beale's Turtle Sacalia bealei Platysternidae 1 Big-headed Turtle Platysternon megacephalum Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Agamidae 1 Changeable Lizard Calotes versicolor Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Long-tailed Skink Eutropis longicaudata Chinese Skink Plestiodon chinensis chinensis Five-striped Blue-tailed Skink Plestiodon elegans Blue-tailed Skink Plestiodon quadrilineatus Vietnamese Five-lined Skink Plestiodon tamdaoensis Slender Forest Skink Scincella modesta Reeve's Smooth Skink Scincella reevesii Brown Forest Skink Sphenomorphus incognitus Indian Forest Skink Sphenomorphus indicus Chinese Waterside Skink Tropidophorus sinicus Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek's Burrowing Lizard Dibamus bogadeki Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko Gekko gecko Bowring's Gecko Hemidactylus bowringii Brook's Gecko* Hemidactylus brookii House Gecko* Hemidactylus