CICE: the Los Alamos Sea Ice Model Documentation and Software User's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nutrient Availability Limits Biological Production in Arctic Sea Ice Melt Ponds
Polar Biol DOI 10.1007/s00300-017-2082-7 ORIGINAL PAPER Nutrient availability limits biological production in Arctic sea ice melt ponds Heidi Louise Sørensen1,2 · Bo Thamdrup1 · Erik Jeppesen2,3,4,7 · Søren Rysgaard2,5,6,7 · Ronnie Nøhr Glud1,2,7,8 Received: 26 February 2016 / Revised: 26 August 2016 / Accepted: 10 January 2017 © The Author(s) 2017. This article is published with open access at Springerlink.com Abstract Every spring and summer melt ponds form at addition compared with their respective controls, with the the surface of polar sea ice and become habitats where bio- largest increase occurring in the enclosures. Separate addi- 3− − logical production may take place. Previous studies report tions of PO4 and NO3 in the enclosures led to interme- a large variability in the productivity, but the causes are diate increases in productivity, suggesting co-limitation of unknown. We investigated if nutrients limit the productiv- nutrients. Bacterial production and the biovolume of cili- ity in these first-year ice melt ponds by adding nutrients to ates, which were the dominant grazers, were positively cor- 3− − 3− three enclosures ([1] PO4 , [2] NO3 , and [3] PO4 and related with primary production, showing a tight coupling − 3− − NO3 ) and one natural melt pond (PO4 and NO3 ), while between primary production and both microbial activity one enclosure and one natural melt pond acted as controls. and ciliate grazing. To our knowledge, this study is the After 7–13 days, Chl a concentrations and cumulative pri- first to ascertain nutrient limitation in melt ponds. We also mary production were between two- and tenfold higher document that the addition of nutrients, although at rela- in the enclosures and natural melt ponds with nutrient tive high concentrations, can stimulate biological produc- tivity at several trophic levels. -

Proton Ordering and Reactivity of Ice
1 Proton ordering and reactivity of ice Zamaan Raza Department of Chemistry University College London Thesis submitted for the degree of Doctor of Philosophy September 2012 2 I, Zamaan Raza, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 3 For Chryselle, without whom I would never have made it this far. 4 I would like to thank my supervisors, Dr Ben Slater and Prof Angelos Michaelides for their patient guidance and help, particularly in light of the fact that I was woefully unprepared when I started. I would also like to express my gratitude to Dr Florian Schiffmann for his indispens- able advice on CP2K and quantum chemistry, Dr Alexei Sokol for various discussions on quantum mechanics, Dr Dario Alfé for his incredibly expensive DMC calculations, Drs Jiri Klimeš and Erlend Davidson for advice on VASP, Matt Watkins for help with CP2K, Christoph Salzmann for discussions on ice, Dr Stefan Bromley for allowing me to work with him in Barcelona and Drs Aron Walsh, Stephen Shevlin, Matthew Farrow and David Scanlon for general help, advice and tolerance. Thanks and also apologies to Stephen Cox, with whom I have collaborated, but have been unable to contribute as much as I should have. Doing a PhD is an isolating experience (more so in the Kathleen Lonsdale building), so I would like to thank my fellow students and friends for making it tolerable: Richard, Tiffany, and Chryselle. Finally, I would like to acknowledge UCL for my funding via a DTA and computing time on Legion, the Materials Chemistry Consortium (MCC) for computing time on HECToR and HPC-Europa2 for the opportunity to work in Barcelona. -

Ice Ic” Werner F
Extent and relevance of stacking disorder in “ice Ic” Werner F. Kuhsa,1, Christian Sippela,b, Andrzej Falentya, and Thomas C. Hansenb aGeoZentrumGöttingen Abteilung Kristallographie (GZG Abt. Kristallographie), Universität Göttingen, 37077 Göttingen, Germany; and bInstitut Laue-Langevin, 38000 Grenoble, France Edited by Russell J. Hemley, Carnegie Institution of Washington, Washington, DC, and approved November 15, 2012 (received for review June 16, 2012) “ ” “ ” A solid water phase commonly known as cubic ice or ice Ic is perfectly cubic ice Ic, as manifested in the diffraction pattern, in frequently encountered in various transitions between the solid, terms of stacking faults. Other authors took up the idea and liquid, and gaseous phases of the water substance. It may form, attempted to quantify the stacking disorder (7, 8). The most e.g., by water freezing or vapor deposition in the Earth’s atmo- general approach to stacking disorder so far has been proposed by sphere or in extraterrestrial environments, and plays a central role Hansen et al. (9, 10), who defined hexagonal (H) and cubic in various cryopreservation techniques; its formation is observed stacking (K) and considered interactions beyond next-nearest over a wide temperature range from about 120 K up to the melt- H-orK sequences. We shall discuss which interaction range ing point of ice. There was multiple and compelling evidence in the needs to be considered for a proper description of the various past that this phase is not truly cubic but composed of disordered forms of “ice Ic” encountered. cubic and hexagonal stacking sequences. The complexity of the König identified what he called cubic ice 70 y ago (11) by stacking disorder, however, appears to have been largely over- condensing water vapor to a cold support in the electron mi- looked in most of the literature. -

A Destabilizing Thermohaline Circulation–Atmosphere–Sea Ice
642 JOURNAL OF CLIMATE VOLUME 12 NOTES AND CORRESPONDENCE A Destabilizing Thermohaline Circulation±Atmosphere±Sea Ice Feedback STEVEN R. JAYNE MIT±WHOI Joint Program in Oceanography, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts JOCHEM MAROTZKE Center for Global Change Science, Department of Earth, Atmospheric and Planetary Sciences, Massachusetts Institute of Technology, Cambridge, Massachusetts 18 November 1996 and 9 March 1998 ABSTRACT Some of the interactions and feedbacks between the atmosphere, thermohaline circulation, and sea ice are illustrated using a simple process model. A simpli®ed version of the annual-mean coupled ocean±atmosphere box model of Nakamura, Stone, and Marotzke is modi®ed to include a parameterization of sea ice. The model includes the thermodynamic effects of sea ice and allows for variable coverage. It is found that the addition of sea ice introduces feedbacks that have a destabilizing in¯uence on the thermohaline circulation: Sea ice insulates the ocean from the atmosphere, creating colder air temperatures at high latitudes, which cause larger atmospheric eddy heat and moisture transports and weaker oceanic heat transports. These in turn lead to thicker ice coverage and hence establish a positive feedback. The results indicate that generally in colder climates, the presence of sea ice may lead to a signi®cant destabilization of the thermohaline circulation. Brine rejection by sea ice plays no important role in this model's dynamics. The net destabilizing effect of sea ice in this model is the result of two positive feedbacks and one negative feedback and is shown to be model dependent. To date, the destabilizing feedback between atmospheric and oceanic heat ¯uxes, mediated by sea ice, has largely been neglected in conceptual studies of thermohaline circulation stability, but it warrants further investigation in more realistic models. -

Tropical Climate
UGAMP: A network of excellence in climate modelling and research Issue 27 October 2003 UGAMP Coordinator: Prof. Julia Slingo [email protected] Newsletter Editor: Dr. Glenn Carver [email protected] Newsletter website: acmsu.nerc.ac.uk/newsletter.html Contents NCAS News . 2 NCAS Websites . 3 NCAS Centres and Facilities . 3 UGAMP Coordinator . 4 CGAM Director . 4 ACMSU Director . 4 HPC Facilities . 5 New areas of UGAMP science 7 Chemistry-climate interactions . 19 Climate variability and predictability . 32 Atmospheric Composition . 48 Tropospheric chemistry and aerosols . 58 Climate Dynamics . 64 Model development . 72 Group News . 78 (for full contents see listing on the inside back cover) NERC Centres for Atmospheric Science, NCAS Alan Thorpe ([email protected]): Director NCAS Since the last UGAMP Newsletter there have been a significant number of NCAS developments relevant to the UK atmospheric science community. These include the following, which are particularly pertinent to the UGAMP community: • NERC have agreed to fund a new directed (new name for thematic) programme called “Surface Ocean – Lower Atmosphere Study” or SOLAS for short. • NERC have agreed to fund a “pump-priming” activity for a proposed new directed programme called Flood Risk from Extreme Events, FREE. The full proposal for FREE will be considered by NERC early in 2004. •NCAS is supporting a project to develop a new chemistry module for the HadGEM model. This is called UK-CHEM and Olaf Morgenstern at ACMSU is collaborating closely with the Hadley Centre on the project. •NCAS is supporting a project to develop the science for a new aerosol module for HadGEM. -

Results from the Implementation of the Elastic Viscous Plastic Sea Ice Rheology in Hadcm3 W
Results from the implementation of the Elastic Viscous Plastic sea ice rheology in HadCM3 W. M. Connolley, A. B. Keen, A. J. Mclaren To cite this version: W. M. Connolley, A. B. Keen, A. J. Mclaren. Results from the implementation of the Elastic Viscous Plastic sea ice rheology in HadCM3. Ocean Science, European Geosciences Union, 2006, 2 (2), pp.201- 211. hal-00298295 HAL Id: hal-00298295 https://hal.archives-ouvertes.fr/hal-00298295 Submitted on 23 Oct 2006 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Ocean Sci., 2, 201–211, 2006 www.ocean-sci.net/2/201/2006/ Ocean Science © Author(s) 2006. This work is licensed under a Creative Commons License. Results from the implementation of the Elastic Viscous Plastic sea ice rheology in HadCM3 W. M. Connolley1, A. B. Keen2, and A. J. McLaren2 1British Antarctic Survey, High Cross, Madingley Road, Cambridge, CB3 0ET, UK 2Met Office Hadley Centre, FitzRoy Road, Exeter, EX1 3PB, UK Received: 13 June 2006 – Published in Ocean Sci. Discuss.: 10 July 2006 Revised: 21 September 2006 – Accepted: 16 October 2006 – Published: 23 October 2006 Abstract. We present results of an implementation of the a full dynamical model incorporating wind stresses and in- Elastic Viscous Plastic (EVP) sea ice dynamics scheme into ternal ice stresses leads to errors in the detailed representa- the Hadley Centre coupled ocean-atmosphere climate model tion of sea ice and limits our confidence in its future predic- HadCM3. -
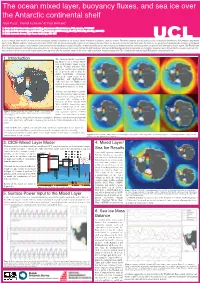
3. CICE-Mixed Layer Model 4. Mixed Layer/ Sea Ice Results 5. Surface
The ocean mixed layer, buoyancy fluxes, and sea ice over the Antarctic continental shelf Alek Petty1, Daniel Feltham2 & Paul Holland3 1. Centre for Polar Observation and Modelling, Department of Earth Sciences, UCL, London, WC1E6BT 2. Centre for Polar Observation and Modelling, Department of Meteorology, Reading University, Reading, RG6 6BB 2. British Antarctic Survey, High Cross, Cambridge, CB3 0ET A sea ice-mixed layer model has been used to investigate regional variations in the surface-driven formation of Antarctic shelf sea waters. The model captures well the expected sea ice thickness distribution, and produces deep mixed layers in the Weddell and Ross shelf seas each winter (1985-2011). By deconstructing the surface power input to the mixed layer, we have shown that the salt/fresh water flux from sea ice growth/melt dominates the evolution of the mixed layer in all shelf sea regions, with a smaller contribution from the mixed layer-surface heat flux. An analysis of the sea ice mass balance has demonstrated the contrasting mean ice growth, melt and export in each region. The Weddell and Ross shelf seas expereince the highest annual ice growth, with a large fraction of this ice exported northwards each year, whereas the Bellingshausen shelf sea experiences the highest annual ice melt, despite the low annual ice growth. Cur- rent work (not shown) is focussed on atmospheric forcing trends and the resultant trends in the sea ice and mixed layer evolution using both ERA-I hindcast forcing and hadGEM2 future climate projections. 1. Introduction The continental shelf seas surround- ing Antarctica are a crucial compo- nent of the Earth’s climate system, with the Weddell and Ross (WR) shelf seas cooling and ventilating the deep ocean and feeding the global thermohaline circulation, whereas the warm waters in the Amundsen and Bellingshausen (AB) shelf seas (see Figure 1) are implicit in the recent ocean-driven melting of the Antarctic ice sheet. -

Glaciers and Their Significance for the Earth Nature - Vladimir M
HYDROLOGICAL CYCLE – Vol. IV - Glaciers and Their Significance for the Earth Nature - Vladimir M. Kotlyakov GLACIERS AND THEIR SIGNIFICANCE FOR THE EARTH NATURE Vladimir M. Kotlyakov Institute of Geography, Russian Academy of Sciences, Moscow, Russia Keywords: Chionosphere, cryosphere, glacial epochs, glacier, glacier-derived runoff, glacier oscillations, glacio-climatic indices, glaciology, glaciosphere, ice, ice formation zones, snow line, theory of glaciation Contents 1. Introduction 2. Development of glaciology 3. Ice as a natural substance 4. Snow and ice in the Nature system of the Earth 5. Snow line and glaciers 6. Regime of surface processes 7. Regime of internal processes 8. Runoff from glaciers 9. Potentialities for the glacier resource use 10. Interaction between glaciation and climate 11. Glacier oscillations 12. Past glaciation of the Earth Glossary Bibliography Biographical Sketch Summary Past, present and future of glaciation are a major focus of interest for glaciology, i.e. the science of the natural systems, whose properties and dynamics are determined by glacial ice. Glaciology is the science at the interfaces between geography, hydrology, geology, and geophysics. Not only glaciers and ice sheets are its subjects, but also are atmospheric ice, snow cover, ice of water basins and streams, underground ice and aufeises (naleds). Ice is a mono-mineral rock. Ten crystal ice variants and one amorphous variety of the ice are known.UNESCO Only the ice-1 variant has been – reve EOLSSaled in the Nature. A cryosphere is formed in the region of interaction between the atmosphere, hydrosphere and lithosphere, and it is characterized bySAMPLE negative or zero temperature. CHAPTERS Glaciology itself studies the glaciosphere that is a totality of snow-ice formations on the Earth's surface. -

Nutrient Availability Limits Biological Production in Arctic Sea Ice Melt Ponds
University of Southern Denmark Nutrient availability limits biological production in Arctic sea ice melt ponds Sørensen, Heidi Louise; Thamdrup, Bo; Jeppesen, Erik; Rysgaard, Søren; Glud, Ronnie N. Published in: Polar Biology DOI: 10.1007/s00300-017-2082-7 Publication date: 2017 Document version: Final published version Document license: CC BY Citation for pulished version (APA): Sørensen, H. L., Thamdrup, B., Jeppesen, E., Rysgaard, S., & Glud, R. N. (2017). Nutrient availability limits biological production in Arctic sea ice melt ponds. Polar Biology, 40(8), 1593-1606. https://doi.org/10.1007/s00300-017-2082-7 Go to publication entry in University of Southern Denmark's Research Portal Terms of use This work is brought to you by the University of Southern Denmark. Unless otherwise specified it has been shared according to the terms for self-archiving. If no other license is stated, these terms apply: • You may download this work for personal use only. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying this open access version If you believe that this document breaches copyright please contact us providing details and we will investigate your claim. Please direct all enquiries to [email protected] Download date: 28. Sep. 2021 Polar Biol (2017) 40:1593–1606 DOI 10.1007/s00300-017-2082-7 ORIGINAL PAPER Nutrient availability limits biological production in Arctic sea ice melt ponds Heidi Louise Sørensen1,2 · Bo Thamdrup1 · Erik Jeppesen2,3,4,7 · Søren Rysgaard2,5,6,7 · Ronnie Nøhr Glud1,2,7,8 Received: 26 February 2016 / Revised: 26 August 2016 / Accepted: 10 January 2017 / Published online: 1 March 2017 © The Author(s) 2017. -

Thermodynamic Stability of Hydrogen Hydrates of Ice Ic and II Structures
PHYSICAL REVIEW B 82, 144105 ͑2010͒ Thermodynamic stability of hydrogen hydrates of ice Ic and II structures Lukman Hakim, Kenichiro Koga, and Hideki Tanaka Department of Chemistry, Faculty of Science, Okayama University, 3-1-1 Tsushima, Kitaku, Okayama 700-8530, Japan ͑Received 6 August 2010; published 13 October 2010͒ The occupancy of hydrogen inside the voids of ice Ic and ice II, which gives two stable hydrogen hydrate compounds at high pressure and temperature, has been examined using a hybrid grand-canonical Monte Carlo simulation in wide ranges of pressure and temperature. The simulation reproduces the maximum hydrogen-to- water molar ratio and gives a detailed description on the hydrogen influence toward the stability of ice structures. A simple theoretical model, which reproduces the simulation results, provides a global phase dia- gram of two-component system in which the phase transitions between various phases can be predicted as a function of pressure, temperature, and chemical composition. A relevant thermodynamic potential and statistical-mechanical ensemble to describe the filled-ice compounds are discussed, from which one can derive two important properties of hydrogen hydrate compounds: the isothermal compressibility and the quantification of thermodynamic stability in term of the chemical potential. DOI: 10.1103/PhysRevB.82.144105 PACS number͑s͒: 64.70.Ja I. INTRODUCTION relative to other water-hydrogen composite phases in a wide range of thermodynamic conditions has been scarcely ex- Storage of hydrogen has been actively investigated to plored. On the other hand, constructing a global phase dia- meet the demand on environmentally clean and efficient gram is a tedious task from experimental view point, consid- hydrogen-based fuel.1 A search for practical hydrogen- ering the number of thermodynamic states to be explored. -

Searching for Crystal-Ice Domains in Amorphous Ices
PHYSICAL REVIEW MATERIALS 2, 075601 (2018) Searching for crystal-ice domains in amorphous ices Fausto Martelli,1,2,* Nicolas Giovambattista,3,4 Salvatore Torquato,2 and Roberto Car2 1IBM Research, Hartree Centre, Daresbury WA4 4AD, United Kingdom 2Department of Chemistry, Princeton University, Princeton, New Jersey 08544, USA 3Department of Physics, Brooklyn College of the City University of New York, Brooklyn, New York 11210, USA 4The Graduate Center of the City University of New York, New York, New York 10016, USA (Received 7 May 2018; published 2 July 2018) Weemploy classical molecular dynamics simulations to investigate the molecular-level structure of water during the isothermal compression of hexagonal ice (Ih) and low-density amorphous (LDA) ice at low temperatures. In both cases, the system transforms to high-density amorphous ice (HDA) via a first-order-like phase transition. We employ a sensitive local order metric (LOM) [F. Martelli et al., Phys. Rev. B 97, 064105 (2018)] that can discriminate among different crystalline and noncrystalline ice structures and is based on the positions of the oxygen atoms in the first- and/or second-hydration shell. Our results confirm that LDA and HDA are indeed amorphous, i.e., they lack polydispersed ice domains. Interestingly, HDA contains a small number of domains that are reminiscent of the unit cell of ice IV,although the hydrogen-bond network (HBN) of these domains differs from the HBN of ice IV. The presence of ice-IV-like domains provides some support to the hypothesis that HDA could be the result of a detour on the HBN rearrangement along the Ih-to-ice-IV pressure-induced transformation. -

Modeling the Ice VI to VII Phase Transition
Modeling the Ice VI to VII Phase Transition Dawn M. King 2009 NSF/REU PROJECT Physics Department University of Notre Dame Advisor: Dr. Kathie E. Newman July 31, 2009 Abstract Ice (solid water) is found in a number of different structures as a function of temperature and pressure. This project focuses on two forms: Ice VI (space group P 42=nmc) and Ice VII (space group Pn3m). An interesting feature of the structural phase transition from VI to VII is that both structures are \self clathrate," which means that each structure has two sublattices which interpenetrate each other but do not directly bond with each other. The goal is to understand the mechanism behind the phase transition; that is, is there a way these structures distort to become the other, or does the transition occur through the breaking of bonds followed by a migration of the water molecules to the new positions? In this project we model the transition first utilizing three dimensional visualization of each structure, then we mathematically develop a common coordinate system for the two structures. The last step will be to create a phenomenological Ising-like spin model of the system to capture the energetics of the transition. It is hoped the spin model can eventually be studied using either molecular dynamics or Monte Carlo simulations. 1 Overview of Ice The known existence of many solid states of water provides insight into the complexity of condensed matter in the universe. The familiarity of ice and the existence of many structures deem ice to be interesting in the development of techniques to understand phase transitions.