Spatial Effects in Microbial Mats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aerobic Respiration
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: + - C6H12O6 + 6 H2O ==> 6 CO2 + 24 H +24 e oxidation electron donor (aka energy source) + - (O2+ 4H + 4e ==> 2H2O) x6 reduction electron acceptor --------------------------------------- C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. - • Aerobic respiration: e acceptor is O2 - • Anaerobic respiration: e acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? carbon source? organic organic CO CO compound 2 compound 2 chemoheterotroph chemoautotroph photoheterotroph photoautotroph e- acceptor? Nitrifying and sulfur- use H O to reduce CO ? oxidizing bacteria 2 2 green non-sulfur and O Other than O 2 2 purple non-sulfur bacteria anoxygenic oxygenic photosynthesis: photosynthesis: green sulfur and most bacteria Organic Inorganic cyanobacteria compound compound purple sulfur bacteria fermentative organism anaerobic respiration: nitrate, sulfate, Fe(III) Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the -

Fermentation and Anaerobic Decomposition in a Hot Spring
Fermentation and anaerobic decomposition in a hot spring microbial mat by Karen Leigh Anderson A thesis submitted in partial fulfillment of requirements for the degree of Master of Science in Microbiology Montana State University © Copyright by Karen Leigh Anderson (1984) Abstract: Fermentation was investigated in a low sulfate hot spring microbial mat (Octopus Spring) according to current models on anaerobic decomposition. The mat was studied to determine what fermentation products accumulated, where in the mat they accumulated, and what factors affected their accumulation. Mat samples were incubated under dark anaerobic conditions to measure accumulation of fermentation products. Acetate and propionate (ca. 3:1) were the major products to accumulate in a 55°,C mat. Other products accumulated to a much lesser extent. Incubation of mat samples of varying thickness showed that fermentation occurred in the top 4mm of the mat. This has interesting implications for fermentative organisms in the mat due to the diurnal changes in mat oxygen concentrations. Fermentation measured in mat samples collected at various temperatures (50°,-70°C) showed acetate and propionate to be the major accumulation products. According to the interspecies hydrogen transfer model, the hydrogen concentration in a system affects the types of fermentation products produced. At a 65° C site, with natural high hydrogen levels, and at a 55°C site, with active methanogenesis, fermentation product accumulation was compared. There was a greater ratio of reduced fermentation products to acetate, with the exception of propionate, at 65°C. Ethanol accumulated at the 65°C site, as did lactate, though to a lesser extent. -

Principles of Ecology Section ●2 Flow of Energy in an Ecosystem
chapter 2 Principles of Ecology section ●2 Flow of Energy in an Ecosystem -!). )DEA Before You Read Autotrophs capture energy, making it available for all If a pet had to survive without your care, how would its diet members of a food web. change? Write your ideas on the lines below. Read about how What You’ll Learn organisms get food and energy in their environment. ■ the fl ow of energy through an ecosystem ■ food chains, food webs, and pyramid models 3TUDY#OACH Read to Learn Make Flash Cards Make a fl ash card for each question Energy in an Ecosystem heading in this section. On the One way to study the interactions within an ecosystem is to back of the fl ash card, write the trace how energy fl ows through the system. All organisms are answer to the question. Use the classifi ed by the way they obtain energy. fl ash cards to review what you have learned. How do autotrophs obtain energy? All green plants and other organisms that produce their own food are the primary producers of food in an ecosystem. They are called autotrophs. An autotroph (AW tuh trohf) is an organism that captures energy from sunlight or inorganic substances to produce food. Autotrophs make energy available for all other organisms in the ecosystem. How do heterotrophs differ from autotrophs? A heterotroph (HE tuh roh trohf), also called a consumer, is an organism that obtains energy by consuming other organisms. A heterotroph that consumes only plants is an herbivore (HUR buh vor). Cows, rabbits, and grasshoppers are herbivores. -

Obecné Znaky Metabolismu + Bioenergetika
Metabolism • General concept of metabolism + Bioenergetics • Cellular respiration (glykolysis + CKC + oxidative phosphorylation) • Sacharide metabolism + photosynthesis • Lipid metabolism • Metabolism of nitrous compounds Obecné znaky metabolismu + bioenergetika BUNĚČNÁ TEORIE Robert Hook (1667) "buňka" 1. Buňky tvoří veškerou živou hmotu (x viry). 2. Veškeré buňky pocházejí z jiných buněk (x samoplození). 3. Informace se předávají z generace na generaci. 4. V buňkách látky podléhají chemickým přeměnám. 5. Buňky reagují na vnější podněty. Otevřené systémy: tok látek, energie a informací dovnitř a ven dynamická rovnováha → ustálený stav Pravá rovnováha → smrt organismu Metabolic types (trofika, trofé = výživa): Energy source: light→ phototroph chemical reaction(redox) → chemotrophs Proton/electron donor acceptor Anorganic Organic Oxygen Other lithotrophs organotrophs aerobic anaerobic (líthos = stone) Fermentace: „disproporcionace“ Např.: C6H12O6 2 CH3-CH(OH)-COOH C6H12O6 2 CH3-CH2-OH + 2CO2 To be continued…….. Carbon source: anorganic → autotrophs organic → heterotrophs Common metabolic types METABOLISM Carbon source Proton source Proton example acceptor photolithotrophs CO2 H2O CO2 Green parts of (autotrophic) plant photolithotrophs Organic comp H2O (CO2) Some (heterotrophic) photosynthetic bacteria Photoorganotrophs Organic comp. Organic comp CO2 Some algae nad (heterotrophic) bacteria chemoorganotrophs Organic comp Organic comp O2 Animals, aerobic aerobic MO 2- Chemoorganotrophs Organic comp Organic comp SO4 Soil anaerobic - Anaerobic -

MIXOTROPHY in FRESHWATER FOOD WEBS a Dissertation
MIXOTROPHY IN FRESHWATER FOOD WEBS A Dissertation Submitted to the Temple University Graduate Board In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY by Sarah B. DeVaul May 2016 Examining Committee Members: Robert W. Sanders, Advisory Chair, Biology Erik E. Cordes, Biology Amy Freestone, Biology Dale Holen, External Member, Penn State Worthington Scranton © Copyright 2016 by Sarah B. DeVaul All Rights Reserved ii ABSTRACT Environmental heterogeneity in both space and time has significant repercussions for community structure and ecosystem processes. Dimictic lakes provide examples of vertically structured ecosystems that oscillate between stable and mixed thermal layers on a seasonal basis. Vertical patterns in abiotic conditions vary during both states, but with differing degrees of variation. For example, during summer thermal stratification there is high spatial heterogeneity in temperature, nutrients, dissolved oxygen and photosynthetically active radiation. The breakdown of stratification and subsequent mixing of the water column in fall greatly reduces the stability of the water column to a vertical gradient in light. Nutrients and biomass that were otherwise constrained to the depths are also suspended, leading to a boom in productivity. Freshwater lakes are teeming with microbial diversity that responds to the dynamic environment in a seemingly predictable manner. Although such patterns have been well studied for nanoplanktonic phototrophic and heterotrophic populations, less work has been done to integrate the influence of mixotrophic nutrition to the protistan assemblage. Phagotrophy by phytoplankton increases the complexity of nutrient and energy flow due to their dual functioning as producers and consumers. The role of mixotrophs in freshwater planktonic communities also varies depending on the relative balance between taxon-specific utilization of carbon and energy sources that ranges widely between phototrophy and heterotrophy. -

Evolution of Oxygenic Photosynthesis
EA44CH24-Fischer ARI 17 May 2016 19:44 ANNUAL REVIEWS Further Click here to view this article's online features: • Download figures as PPT slides • Navigate linked references • Download citations Evolution of Oxygenic • Explore related articles • Search keywords Photosynthesis Woodward W. Fischer, James Hemp, and Jena E. Johnson Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: wfi[email protected] Annu. Rev. Earth Planet. Sci. 2016. 44:647–83 Keywords First published online as a Review in Advance on Great Oxidation Event, photosystem II, chlorophyll, oxygen evolving May 11, 2016 complex, molecular evolution, Precambrian The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The origin of oxygenic photosynthesis was the most important metabolic 10.1146/annurev-earth-060313-054810 innovation in Earth history. It allowed life to generate energy and reducing Copyright c 2016 by Annual Reviews. power directly from sunlight and water, freeing it from the limited resources All rights reserved of geochemically derived reductants. This greatly increased global primary productivity and restructured ecosystems. The release of O2 as an end prod- Access provided by California Institute of Technology on 07/14/16. For personal use only. Annu. Rev. Earth Planet. Sci. 2016.44:647-683. Downloaded from www.annualreviews.org uct of water oxidation led to the rise of oxygen, which dramatically altered the redox state of Earth’s atmosphere and oceans and permanently changed all major biogeochemical cycles. Furthermore, the biological availability of O2 allowed for the evolution of aerobic respiration and novel biosynthetic pathways, facilitating much of the richness we associate with modern biology, including complex multicellularity. -

Chapter 4: PROKARYOTIC DIVERSITY
Chapter 4: PROKARYOTIC DIVERSITY 1. Prokaryote Habitats, Relationships & Biomes 2. Proteobacteria 3. Gram-negative and Phototropic Non-Proteobacteria 4. Gram-Positive Bacteria 5. Deeply Branching Bacteria 6. Archaea 1. Prokaryote Habitats, Relationships & Biomes Important Metabolic Terminology Oxygen tolerance/usage: aerobic – requires or can use oxygen (O2) anaerobic – does not require or cannot tolerate O2 Energy usage: phototroph – uses light as an energy source • all photosynthetic organisms chemotroph – acquires energy from organic or inorganic molecules • organotrophs – get energy from organic molecules • lithotrophs – get energy from inorganic molecules …more Important Terminology Carbon Source: autotroph – uses CO2 as a carbon source • e.g., photoautotrophs or chemoautotrophs heterotroph – requires an organic carbon source • e.g., chemoheterotroph – gets energy & carbon from organic molecules Oligotrophs require few nutrients, the opposite of eutrophs or copiotrophs Facultative vs Obligate (or Strict): facultative – “able to, but not requiring” • e.g., facultative anaerobes can survive w/ or w/o O2 obligate – “absolutely requires” • e.g., obligate anaerobes cannot survive in O2 Symbiotic Relationships Symbiotic relationships (close, direct interactions) between different organisms in nature are of several types: • e.g., humans have beneficial bacteria in their digestive tracts that also benefit from the food we eat (mutualism) Microbiomes All the microorganisms that inhabit a particular organism or environment (e.g., human or -

Microbial Diversity of Co-Occurring Heterotrophs in Cultures of Marine Picocyanobacteria Sean M
Kearney et al. Environmental Microbiome (2021) 16:1 Environmental Microbiome https://doi.org/10.1186/s40793-020-00370-x RESEARCH ARTICLE Open Access Microbial diversity of co-occurring heterotrophs in cultures of marine picocyanobacteria Sean M. Kearney*† , Elaina Thomas† , Allison Coe and Sallie W. Chisholm* Abstract Background: The cyanobacteria Prochlorococcus and Synechococcus are responsible for around 10% of global net primary productivity, serving as part of the foundation of marine food webs. Heterotrophic bacteria are often co- isolated with these picocyanobacteria in seawater enrichment cultures that contain no added organic carbon; heterotrophs grow on organic carbon supplied by the photolithoautotrophs. For examining the selective pressures shaping autotroph/heterotroph interactions, we have made use of unialgal enrichment cultures of Prochlorococcus and Synechococcus maintained for hundreds to thousands of generations in the lab. We examine the diversity of heterotrophs in 74 enrichment cultures of these picocyanobacteria obtained from diverse areas of the global oceans. Results: Heterotroph community composition differed between clades and ecotypes of the autotrophic ‘hosts’ but there was significant overlap in heterotroph community composition across these cultures. Collectively, the cultures were comprised of many shared taxa, even at the genus level. Yet, observed differences in community composition were associated with time since isolation, location, depth, and methods of isolation. The majority of heterotrophs in the cultures are rare in the global ocean, but enrichment conditions favor the opportunistic outgrowth of these rare bacteria. However, we found a few examples, such as bacteria in the family Rhodobacteraceae, of heterotrophs that were ubiquitous and abundant in cultures and in the global oceans. -

7.014 Lectures 16 &17: the Biosphere & Carbon and Energy Metabolism
MIT Department of Biology 7.014 Introductory Biology, Spring 2005 7.014 Lectures 16 &17: The Biosphere & Carbon and Energy Metabolism Simplified Summary of Microbial Metabolism The metabolism of different types of organisms drives the biogeochemical cycles of the biosphere. Balanced oxidation and reduction reactions keep the system from “running down”. All living organisms can be ordered into two groups1, autotrophs and heterotrophs, according to what they use as their carbon source. Within these groups the metabolism of organisms can be further classified according to their source of energy and electrons. Autotrophs: Those organisms get their energy from light (photoautotrophs) or reduced inorganic compounds (chemoautotrophs), and their carbon from CO2 through one of the following processes: Photosynthesis (aerobic) — Light energy used to reduce CO2 to organic carbon using H2O as a source of electrons. O2 evolved from splitting H2O. (Plants, algae, cyanobacteria) Bacterial Photosynthesis (anaerobic) — Light energy used to reduce CO2 to organic carbon (same as photosynthesis). H2S is used as the electron donor instead of H2O. (e.g. purple sulfur bacteria) Chemosynthesis (aerobic) — Energy from the oxidation of inorganic molecules is used to reduce CO2 to organic carbon (bacteria only). -2 e.g. sulfur oxidizing bacteria H2S → S → SO4 + - • nitrifying bacteria NH4 → NO2 → NO3 iron oxidizing bacteria Fe+2 → Fe+3 methane oxidizing bacteria (methanotrophs) CH4 → CO2 Heterotrophs: These organisms get their energy and carbon from organic compounds (supplied by autotrophs through the food web) through one or more of the following processes: Aerobic Respiration (aerobic) ⎯ Oxidation of organic compounds to CO2 and H2O, yielding energy for biological work. -

Extremophile Cards
hloroexus aurantiacus “Color-changer, green to orange” einococcus radiodurans yrococcus furiosus Domain: Bacteria “Terrible berry, survives radiation” “Raging reball” Habitat: Hot springs around the world, including Domain: Bacteria Domain: Archaea Yellowstone National Park. Habitat: Widespread, including deserts, hot springs, Habitat: Hydrothermal vents in ocean oor; rst Energy Source: Mixotroph (phototroph and high mountains, polar regions and animal gut. discovered near volcanic islands in Italy. chemotroph). Mainly uses light energy from the sun. In the dark, can use chemical energy from inorganic Energy Source: Chemotroph. Uses chemical energy Energy Source: Chemotroph. Uses chemical energy compounds (sulphur and hydrogen). from organic compounds. from organic compounds. Challenges to Life: Heat, UV exposure, changing Challenges to Life: Dehydration, cold, acidic pH, Challenges to Life: Heat, pressure, changes in levels of light and oxygen radiation, oxidative damage oxygen levels when vent uids contact sea water Adaptation: Can rebuild its genome, after radiation Adaptation: A protein called reverse gyrase helps breaks it into hundreds of fragments, using a unique maintain DNA structure in extreme heat. High Adaptation: Can do both photosynthesis (like DNA-repair mechanism. Produces antioxidants that temperatures tend to unzip double-stranded DNA. plants) and respiration (like animals), allowing it to protect proteins from radiation damage. Reverse gyrase twists DNA so much it can’t unravel. survive a range of light and oxygen levels. It is green during photosynthesis and orange during respiration. Talent: Radioresistant. Survives short doses of Talent: Hyperthermophile. rives in extremely hot radiation up to 5,000 Gy. Grows under constant temperatures. Grows best at 100°C (212°F), Talent: ermophile. -
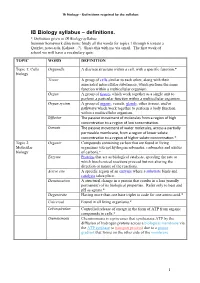
IB Biology Syllabus – Definitions
IB Biology – Definitions required by the syllabus IB Biology syllabus – definitions. * Definition given in IB Biology syllabus Summer homework directions: Study all the words for topics 1 through 6 (create a Quizlet, notecards, Kahoot…?). Share this with me via email. The first week of school we will have a vocabulary quiz. TOPIC WORD DEFINITION Topic 1: Cells Organelle A discrete structure within a cell, with a specific function.* biology Tissue A group of cells similar to each other, along with their associated intercellular substances, which perform the same function within a multicellular organism. Organ A group of tissues which work together as a single unit to perform a particular function within a multicellular organism. Organ system A group of organs, vessels, glands, other tissues, and/or pathways which work together to perform a body function within a multicellular organism. Diffusion The passive movement of molecules from a region of high concentration to a region of low concentration. Osmosis The passive movement of water molecules, across a partially permeable membrane, from a region of lower solute concentration to a region of higher solute concentration.* Topic 2: Organic Compounds containing carbon that are found in living Molecular organisms (except hydrogencarbonates, carbonates and oxides biology of carbon).* Enzyme Proteins that act as biological catalysts, speeding the rate at which biochemical reactions proceed but not altering the direction or nature of the reactions. Active site A specific region of an enzyme where a substrate binds and catalysis takes place. Denaturation A structural change in a protein that results in a loss (usually permanent) of its biological properties. -

Identifying the Missing Steps of the Autotrophic 3-Hydroxypropionate
Identifying the missing steps of the autotrophic SEE COMMENTARY 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus Jan Zarzyckia, Volker Brechtb, Michael Mu¨ llerb, and Georg Fuchsa,1 aMikrobiologie, Fakulta¨t fu¨ r Biologie, Universita¨t Freiburg, Scha¨nzlestrasse 1, D-79104 Freiburg, Germany; and bInstitut fu¨r Pharmazeutische Wissenschaften, Universita¨t Freiburg, Albertstrasse 25, D-79104 Freiburg, Germany Edited by Donald A. Bryant, Pennsylvania State University, University Park, PA, and accepted by the Editorial Board October 10, 2009 (received for review July 27, 2009) The phototrophic bacterium Chloroflexus aurantiacus uses a yet metabolite, from which all building blocks for polymers can be unsolved 3-hydroxypropionate cycle for autotrophic CO2 fixation. derived. It starts from acetyl-CoA, with acetyl-CoA and propionyl-CoA In nature 6 mechanisms to assimilate CO2 into cell material have carboxylases acting as carboxylating enzymes. In a first cycle, been established (1). In the reductive pentose phosphate (Calvin– (S)-malyl-CoA is formed from acetyl-CoA and 2 molecules of bicar- Benson–Bassham) cycle discovered about 50 years ago, CO2 reacts bonate. (S)-Malyl-CoA cleavage releases the CO2 fixation product with a 5-carbon sugar yielding 2 carboxylic acids from which the glyoxylate and regenerates the starting molecule acetyl-CoA. Here sugar is regenerated (2). This cycle operates in plants, algae, we complete the missing steps devoted to glyoxylate assimilation. cyanobacteria, and in aerobic or facultative anaerobic proteobac- In a second cycle, glyoxylate is combined with propionyl-CoA, an teria. The presence of the key enzyme, ribulose 1,5-bisphosphate intermediate of the first cycle, to form -methylmalyl-CoA.