Aerobic Respiration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
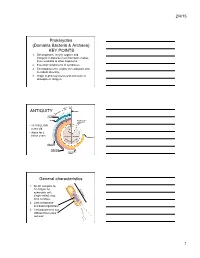
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

Nitrogen and Phosphorus Limitation of Oceanic Microbial Growth During Spring in the Gulf of Aqaba
Vol. 56: 227–239, 2009 AQUATIC MICROBIAL ECOLOGY Printed September 2009 doi: 10.3354/ame01357 Aquat Microb Ecol Published online August 27, 2009 Contribution to AME Special 2 ‘Progress and perspectives in aquatic primary productivity’ OPEN ACCESS Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba David J. Suggett1,*, Noga Stambler2, Ondrej Prá$il3, Zbigniew Kolber4, Antonietta Quigg5, Evaristo Vázquez-Domínguez6, Tamar Zohary7, Tom Berman7, David Iluz8, Orly Levitan2, Tracy Lawson1, Efrat Meeder9,10, Boaz Lazar9,10, Edo Bar-Zeev2, Hana Medova3, Ilana Berman-Frank8 1Department of Biological Sciences, University of Essex, Colchester CO4 3SQ, UK 2Department of Geography and Environment, Bar-Ilan University, Ramat-Gan 52900, Israel 3Photosynthesis Laboratory, Institute of Microbiology ASCR, Opatovicky mlyn, 379 81 Trˇ ebonˇ, Czech Republic 4Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 5Department of Marine Biology and Oceanography, Texas A&M University, Galveston, Texas 77551, USA 6CSIC, Institut de Ciències del Mar, Passeig Marítim de la Barceloneta 39-43, 08003 Barcelona, Spain 7Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research, PO Box 447, Migdal 14950, Israel 8The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel 9The Interuniversity Institute for Marine Sciences Coral Beach, PO Box 469, 88103 Eilat, Israel 10Institute of Earth Sciences, The Hebrew University Edmond J. Safra Campus, Jerusalem 91904, Israel ABSTRACT: Bioassay experiments were performed to identify how growth of key groups within the mi- crobial community was simultaneously limited by nutrient (nitrogen and phosphorus) availability during spring in the Gulf of Aqaba’s oceanic waters. -

Fermentation and Anaerobic Decomposition in a Hot Spring
Fermentation and anaerobic decomposition in a hot spring microbial mat by Karen Leigh Anderson A thesis submitted in partial fulfillment of requirements for the degree of Master of Science in Microbiology Montana State University © Copyright by Karen Leigh Anderson (1984) Abstract: Fermentation was investigated in a low sulfate hot spring microbial mat (Octopus Spring) according to current models on anaerobic decomposition. The mat was studied to determine what fermentation products accumulated, where in the mat they accumulated, and what factors affected their accumulation. Mat samples were incubated under dark anaerobic conditions to measure accumulation of fermentation products. Acetate and propionate (ca. 3:1) were the major products to accumulate in a 55°,C mat. Other products accumulated to a much lesser extent. Incubation of mat samples of varying thickness showed that fermentation occurred in the top 4mm of the mat. This has interesting implications for fermentative organisms in the mat due to the diurnal changes in mat oxygen concentrations. Fermentation measured in mat samples collected at various temperatures (50°,-70°C) showed acetate and propionate to be the major accumulation products. According to the interspecies hydrogen transfer model, the hydrogen concentration in a system affects the types of fermentation products produced. At a 65° C site, with natural high hydrogen levels, and at a 55°C site, with active methanogenesis, fermentation product accumulation was compared. There was a greater ratio of reduced fermentation products to acetate, with the exception of propionate, at 65°C. Ethanol accumulated at the 65°C site, as did lactate, though to a lesser extent. -

Obecné Znaky Metabolismu + Bioenergetika
Metabolism • General concept of metabolism + Bioenergetics • Cellular respiration (glykolysis + CKC + oxidative phosphorylation) • Sacharide metabolism + photosynthesis • Lipid metabolism • Metabolism of nitrous compounds Obecné znaky metabolismu + bioenergetika BUNĚČNÁ TEORIE Robert Hook (1667) "buňka" 1. Buňky tvoří veškerou živou hmotu (x viry). 2. Veškeré buňky pocházejí z jiných buněk (x samoplození). 3. Informace se předávají z generace na generaci. 4. V buňkách látky podléhají chemickým přeměnám. 5. Buňky reagují na vnější podněty. Otevřené systémy: tok látek, energie a informací dovnitř a ven dynamická rovnováha → ustálený stav Pravá rovnováha → smrt organismu Metabolic types (trofika, trofé = výživa): Energy source: light→ phototroph chemical reaction(redox) → chemotrophs Proton/electron donor acceptor Anorganic Organic Oxygen Other lithotrophs organotrophs aerobic anaerobic (líthos = stone) Fermentace: „disproporcionace“ Např.: C6H12O6 2 CH3-CH(OH)-COOH C6H12O6 2 CH3-CH2-OH + 2CO2 To be continued…….. Carbon source: anorganic → autotrophs organic → heterotrophs Common metabolic types METABOLISM Carbon source Proton source Proton example acceptor photolithotrophs CO2 H2O CO2 Green parts of (autotrophic) plant photolithotrophs Organic comp H2O (CO2) Some (heterotrophic) photosynthetic bacteria Photoorganotrophs Organic comp. Organic comp CO2 Some algae nad (heterotrophic) bacteria chemoorganotrophs Organic comp Organic comp O2 Animals, aerobic aerobic MO 2- Chemoorganotrophs Organic comp Organic comp SO4 Soil anaerobic - Anaerobic -

Bacterial Community Structure in a Sympagic Habitat Expanding with Global Warming: Brackish Ice Brine at 85€“90 °N
The ISME Journal (2019) 13:316–333 https://doi.org/10.1038/s41396-018-0268-9 ARTICLE Bacterial community structure in a sympagic habitat expanding with global warming: brackish ice brine at 85–90 °N 1,11 1,2 3 4 5,8 Beatriz Fernández-Gómez ● Beatriz Díez ● Martin F. Polz ● José Ignacio Arroyo ● Fernando D. Alfaro ● 1 1 2,6 5 4,7 Germán Marchandon ● Cynthia Sanhueza ● Laura Farías ● Nicole Trefault ● Pablo A. Marquet ● 8,9 10 10 Marco A. Molina-Montenegro ● Peter Sylvander ● Pauline Snoeijs-Leijonmalm Received: 12 February 2018 / Revised: 11 June 2018 / Accepted: 24 July 2018 / Published online: 18 September 2018 © International Society for Microbial Ecology 2018 Abstract Larger volumes of sea ice have been thawing in the Central Arctic Ocean (CAO) during the last decades than during the past 800,000 years. Brackish brine (fed by meltwater inside the ice) is an expanding sympagic habitat in summer all over the CAO. We report for the first time the structure of bacterial communities in this brine. They are composed of psychrophilic extremophiles, many of them related to phylotypes known from Arctic and Antarctic regions. Community structure displayed strong habitat segregation between brackish ice brine (IB; salinity 2.4–9.6) and immediate sub-ice seawater (SW; – 1234567890();,: 1234567890();,: salinity 33.3 34.9), expressed at all taxonomic levels (class to genus), by dominant phylotypes as well as by the rare biosphere, and with specialists dominating IB and generalists SW. The dominant phylotypes in IB were related to Candidatus Aquiluna and Flavobacterium, those in SW to Balneatrix and ZD0405, and those shared between the habitats to Halomonas, Polaribacter and Shewanella. -

MIXOTROPHY in FRESHWATER FOOD WEBS a Dissertation
MIXOTROPHY IN FRESHWATER FOOD WEBS A Dissertation Submitted to the Temple University Graduate Board In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY by Sarah B. DeVaul May 2016 Examining Committee Members: Robert W. Sanders, Advisory Chair, Biology Erik E. Cordes, Biology Amy Freestone, Biology Dale Holen, External Member, Penn State Worthington Scranton © Copyright 2016 by Sarah B. DeVaul All Rights Reserved ii ABSTRACT Environmental heterogeneity in both space and time has significant repercussions for community structure and ecosystem processes. Dimictic lakes provide examples of vertically structured ecosystems that oscillate between stable and mixed thermal layers on a seasonal basis. Vertical patterns in abiotic conditions vary during both states, but with differing degrees of variation. For example, during summer thermal stratification there is high spatial heterogeneity in temperature, nutrients, dissolved oxygen and photosynthetically active radiation. The breakdown of stratification and subsequent mixing of the water column in fall greatly reduces the stability of the water column to a vertical gradient in light. Nutrients and biomass that were otherwise constrained to the depths are also suspended, leading to a boom in productivity. Freshwater lakes are teeming with microbial diversity that responds to the dynamic environment in a seemingly predictable manner. Although such patterns have been well studied for nanoplanktonic phototrophic and heterotrophic populations, less work has been done to integrate the influence of mixotrophic nutrition to the protistan assemblage. Phagotrophy by phytoplankton increases the complexity of nutrient and energy flow due to their dual functioning as producers and consumers. The role of mixotrophs in freshwater planktonic communities also varies depending on the relative balance between taxon-specific utilization of carbon and energy sources that ranges widely between phototrophy and heterotrophy. -

Evolution of Oxygenic Photosynthesis
EA44CH24-Fischer ARI 17 May 2016 19:44 ANNUAL REVIEWS Further Click here to view this article's online features: • Download figures as PPT slides • Navigate linked references • Download citations Evolution of Oxygenic • Explore related articles • Search keywords Photosynthesis Woodward W. Fischer, James Hemp, and Jena E. Johnson Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: wfi[email protected] Annu. Rev. Earth Planet. Sci. 2016. 44:647–83 Keywords First published online as a Review in Advance on Great Oxidation Event, photosystem II, chlorophyll, oxygen evolving May 11, 2016 complex, molecular evolution, Precambrian The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The origin of oxygenic photosynthesis was the most important metabolic 10.1146/annurev-earth-060313-054810 innovation in Earth history. It allowed life to generate energy and reducing Copyright c 2016 by Annual Reviews. power directly from sunlight and water, freeing it from the limited resources All rights reserved of geochemically derived reductants. This greatly increased global primary productivity and restructured ecosystems. The release of O2 as an end prod- Access provided by California Institute of Technology on 07/14/16. For personal use only. Annu. Rev. Earth Planet. Sci. 2016.44:647-683. Downloaded from www.annualreviews.org uct of water oxidation led to the rise of oxygen, which dramatically altered the redox state of Earth’s atmosphere and oceans and permanently changed all major biogeochemical cycles. Furthermore, the biological availability of O2 allowed for the evolution of aerobic respiration and novel biosynthetic pathways, facilitating much of the richness we associate with modern biology, including complex multicellularity. -

Chapter 4: PROKARYOTIC DIVERSITY
Chapter 4: PROKARYOTIC DIVERSITY 1. Prokaryote Habitats, Relationships & Biomes 2. Proteobacteria 3. Gram-negative and Phototropic Non-Proteobacteria 4. Gram-Positive Bacteria 5. Deeply Branching Bacteria 6. Archaea 1. Prokaryote Habitats, Relationships & Biomes Important Metabolic Terminology Oxygen tolerance/usage: aerobic – requires or can use oxygen (O2) anaerobic – does not require or cannot tolerate O2 Energy usage: phototroph – uses light as an energy source • all photosynthetic organisms chemotroph – acquires energy from organic or inorganic molecules • organotrophs – get energy from organic molecules • lithotrophs – get energy from inorganic molecules …more Important Terminology Carbon Source: autotroph – uses CO2 as a carbon source • e.g., photoautotrophs or chemoautotrophs heterotroph – requires an organic carbon source • e.g., chemoheterotroph – gets energy & carbon from organic molecules Oligotrophs require few nutrients, the opposite of eutrophs or copiotrophs Facultative vs Obligate (or Strict): facultative – “able to, but not requiring” • e.g., facultative anaerobes can survive w/ or w/o O2 obligate – “absolutely requires” • e.g., obligate anaerobes cannot survive in O2 Symbiotic Relationships Symbiotic relationships (close, direct interactions) between different organisms in nature are of several types: • e.g., humans have beneficial bacteria in their digestive tracts that also benefit from the food we eat (mutualism) Microbiomes All the microorganisms that inhabit a particular organism or environment (e.g., human or -

7.014 Lectures 16 &17: the Biosphere & Carbon and Energy Metabolism
MIT Department of Biology 7.014 Introductory Biology, Spring 2005 7.014 Lectures 16 &17: The Biosphere & Carbon and Energy Metabolism Simplified Summary of Microbial Metabolism The metabolism of different types of organisms drives the biogeochemical cycles of the biosphere. Balanced oxidation and reduction reactions keep the system from “running down”. All living organisms can be ordered into two groups1, autotrophs and heterotrophs, according to what they use as their carbon source. Within these groups the metabolism of organisms can be further classified according to their source of energy and electrons. Autotrophs: Those organisms get their energy from light (photoautotrophs) or reduced inorganic compounds (chemoautotrophs), and their carbon from CO2 through one of the following processes: Photosynthesis (aerobic) — Light energy used to reduce CO2 to organic carbon using H2O as a source of electrons. O2 evolved from splitting H2O. (Plants, algae, cyanobacteria) Bacterial Photosynthesis (anaerobic) — Light energy used to reduce CO2 to organic carbon (same as photosynthesis). H2S is used as the electron donor instead of H2O. (e.g. purple sulfur bacteria) Chemosynthesis (aerobic) — Energy from the oxidation of inorganic molecules is used to reduce CO2 to organic carbon (bacteria only). -2 e.g. sulfur oxidizing bacteria H2S → S → SO4 + - • nitrifying bacteria NH4 → NO2 → NO3 iron oxidizing bacteria Fe+2 → Fe+3 methane oxidizing bacteria (methanotrophs) CH4 → CO2 Heterotrophs: These organisms get their energy and carbon from organic compounds (supplied by autotrophs through the food web) through one or more of the following processes: Aerobic Respiration (aerobic) ⎯ Oxidation of organic compounds to CO2 and H2O, yielding energy for biological work. -

An Evolutionary Optimization of a Rhodopsin-Based Phototrophic
Kim et al. Microb Cell Fact (2017) 16:111 DOI 10.1186/s12934-017-0725-6 Microbial Cell Factories RESEARCH Open Access An evolutionary optimization of a rhodopsin‑based phototrophic metabolism in Escherichia coli Hyun Aaron Kim1,7†, Hyun Ju Kim2†, Jihoon Park3, Ah Reum Choi4, Kyoo Heo6, Haeyoung Jeong5, Kwang‑Hwan Jung4, Yeong‑Jae Seok6, Pil Kim3* and Sang Jun Lee2* Abstract Background: The expression of the Gloeobacter rhodopsin (GR) in a chemotrophic Escherichia coli enables the light- driven phototrophic energy generation. Adaptive laboratory evolution has been used for acquiring desired pheno‑ type of microbial cells and for the elucidation of basic mechanism of molecular evolution. To develop an optimized strain for the artifcially acquired phototrophic metabolism, an ancestral E. coli expressing GR was adaptively evolved in a chemostat reactor with constant illumination and limited glucose conditions. This study was emphasized at an unexpected genomic mutation contributed to the improvement of microbial performance. Results: During the chemostat culture, increase of cell size was observed, which were distinguished from that of the typical rod-shaped ancestral cells. A descendant ET5 strain was randomly isolated from the chemostat culture at 88-days. The phototrophic growth and the light-induced proton pumping of the ET5 strain were twofold and eightfold greater, respectively, than those of the ancestral E. coli strain. Single point mutation of C1082A at dgcQ gene (encoding diguanylate cyclase, also known as the yedQ gene) in the chromosome of ET5 strain was identifed from whole genome sequencing analysis. An ancestral E. coli complemented with the same dgcQ mutation from the ET5 was repeated the subsequently enhancements of light-driven phototrophic growth and proton pumping.