Module 3 Microbial Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
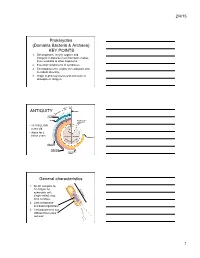
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Microbial Community Composition and Function Beneath Temperate Trees Exposed to Elevated Atmospheric Carbon Dioxide and Ozone
Oecologia (2002) 131:236–244 DOI 10.1007/s00442-002-0868-x ECOSYSTEMS ECOLOGY Rebecca L. Phillips · Donald R. Zak William E. Holmes · David C. White Microbial community composition and function beneath temperate trees exposed to elevated atmospheric carbon dioxide and ozone Received: 1 August 2001 / Accepted: 13 December 2001 / Published online: 14 February 2002 © Springer-Verlag 2002 Abstract We hypothesized that changes in plant growth activity and microbial metabolism of cellobiose, and that resulting from atmospheric CO2 and O3 enrichment microbial processes under early-successional aspen and would alter the flow of C through soil food webs and birch species were more strongly affected by CO2 and O3 that this effect would vary with tree species. To test this enrichment than those under late-successional maple. idea, we traced the course of C through the soil microbial community using soils from the free-air CO2 and O3 Keywords Soil microorganisms · enrichment site in Rhinelander, Wisconsin. We added Carbon-13-phospholipid fatty acid analysis · either 13C-labeled cellobiose or 13C-labeled N-acetylglu- Elevated carbon dioxide · Elevated ozone · cosamine to soils collected beneath ecologically distinct Soil carbon cycling temperate trees exposed for 3 years to factorial CO2 –1 (ambient and 200 µl l above ambient) and O3 (ambient and 20 µl l–1 above ambient) treatments. For both labeled Introduction substrates, recovery of 13C in microbial respiration increased beneath plants grown under elevated CO2 by Human activity has increased the concentration of CO2 29% compared to ambient; elevated O3 eliminated this and O3 in the earth’s troposphere (Barnola et al. -

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

The Electron Transport Chain in Anaerobically Functioning Eukaryotes
rl BIOCHIMICA ET BIOPHYSICA ACTA II BBt ELSEVIER Biochimica et Biophysica Acta, 1365 (1998) 71-78 The electron transport chain in anaerobically functioning eukaryotes Aloysius G.M. Tielens*, Jaap J. Van Hellemond Laboratory of Veterinary Biochemistry and Institute for Biomembranes, Utrecht University, P.O. Box 80176, 3508 TD Utrecht, The Netherlands Received 27 January 1998; received in revised form 26 February 1998; accepted 2 March 1998 Abstract Many lower eukaryotes can survive anaerobic conditions via a fermentation pathway that involves the use of the reduction of endogenously produced fumarate as electron sink. This fumarate reduction is linked to electron transport in an especially adapted, anaerobically functioning electron-transport chain. An aerobic energy metabolism with Krebs cycle activity is accompanied by electron transfer from succinate to ubiquinone via complex II of the respiratory chain. On the other hand, in an anaerobic metabolism, where fumarate functions as terminal electron acceptor, electrons are transferred from rhodoquinone to fumarate, which is the reversed direction. Ubiquinone cannot replace rhodoquinone in the process of fumarate reduction in vivo, as ubiquinone can only accept electrons from complex II and cannot donate them to fumarate. Rhodoquinone, with its lower redox potential than ubiquinone, is capable of donating electrons to fumarate. Eukaryotic fumarate reductases were shown to interact with rhodoquinone (a benzoquinone), whereas most prokaryotic fumarate reductases interact with the naphtoquinones mena- quinone and demethylmenaquinone. Fumarate reductase, the enzyme essential for the anaerobic functioning of many eukaryotes, is structurally very similar to succinate dehydrogenase, the Krebs cycle enzyme catalysing the reverse reaction. In prokaryotes these enzymes are differentially expressed depending on the external conditions. -

Thermophilic Lithotrophy and Phototrophy in an Intertidal, Iron-Rich, Geothermal Spring 2 3 Lewis M
bioRxiv preprint doi: https://doi.org/10.1101/428698; this version posted September 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Thermophilic Lithotrophy and Phototrophy in an Intertidal, Iron-rich, Geothermal Spring 2 3 Lewis M. Ward1,2,3*, Airi Idei4, Mayuko Nakagawa2,5, Yuichiro Ueno2,5,6, Woodward W. 4 Fischer3, Shawn E. McGlynn2* 5 6 1. Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138 USA 7 2. Earth-Life Science Institute, Tokyo Institute of Technology, Meguro, Tokyo, 152-8550, Japan 8 3. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 9 91125 USA 10 4. Department of Biological Sciences, Tokyo Metropolitan University, Hachioji, Tokyo 192-0397, 11 Japan 12 5. Department of Earth and Planetary Sciences, Tokyo Institute of Technology, Meguro, Tokyo, 13 152-8551, Japan 14 6. Department of Subsurface Geobiological Analysis and Research, Japan Agency for Marine-Earth 15 Science and Technology, Natsushima-cho, Yokosuka 237-0061, Japan 16 Correspondence: [email protected] or [email protected] 17 18 Abstract 19 Hydrothermal systems, including terrestrial hot springs, contain diverse and systematic 20 arrays of geochemical conditions that vary over short spatial scales due to progressive interaction 21 between the reducing hydrothermal fluids, the oxygenated atmosphere, and in some cases 22 seawater. At Jinata Onsen, on Shikinejima Island, Japan, an intertidal, anoxic, iron- and 23 hydrogen-rich hot spring mixes with the oxygenated atmosphere and sulfate-rich seawater over 24 short spatial scales, creating an enormous range of redox environments over a distance ~10 m. -

Biol 1020: Photosynthesis
Chapter 10: Photosynthesis Energy and Carbon Sources Electromagnetic Spectrum and Light Chloroplasts Photosynthesis Overview Light Reactions C3 Cycle Photorespiration Supplemental Carbon Fixation: C4 and CAM pathways . • List and differentiate the 4 possible groups of organisms based on how they obtain energy and useful carbon. Classification by Energy and Carbon Sources energy source chemotrophs can only get energy directly from chemical compounds phototrophs can get energy directly from light (these organisms can use chemical compounds as energy sources as well) . Classification by Energy and Carbon Sources carbon source autotrophs can fix carbon dioxide, thus they can use CO2 as a carbon source heterotrophs cannot fix CO2; they use organic molecules from other organisms as a carbon source . Classification by Energy and Carbon Sources combined, these leads to 4 possible groups: photoautotrophs – carry out photosynthesis use light energy to fix CO2 store energy in chemical bonds of organic molecules includes green plants, algae, and some bacteria photoheterotrophs – use light energy but cannot fix CO2; some nonsulfur purple bacteria chemoautotrophs – obtain energy from reduced inorganic molecules and use some of it to fix CO2; some bacteria chemoheterotrophs – use organic molecules as both carbon and energy sources dependent completely on other organisms for energy capture and carbon fixation includes all animals, all fungi, most protists, and most bacteria . • List and differentiate the 4 possible groups of -

Aerobic Respiration
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: + - C6H12O6 + 6 H2O ==> 6 CO2 + 24 H +24 e oxidation electron donor (aka energy source) + - (O2+ 4H + 4e ==> 2H2O) x6 reduction electron acceptor --------------------------------------- C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. - • Aerobic respiration: e acceptor is O2 - • Anaerobic respiration: e acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? carbon source? organic organic CO CO compound 2 compound 2 chemoheterotroph chemoautotroph photoheterotroph photoautotroph e- acceptor? Nitrifying and sulfur- use H O to reduce CO ? oxidizing bacteria 2 2 green non-sulfur and O Other than O 2 2 purple non-sulfur bacteria anoxygenic oxygenic photosynthesis: photosynthesis: green sulfur and most bacteria Organic Inorganic cyanobacteria compound compound purple sulfur bacteria fermentative organism anaerobic respiration: nitrate, sulfate, Fe(III) Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the -

Nitrogen and Phosphorus Limitation of Oceanic Microbial Growth During Spring in the Gulf of Aqaba
Vol. 56: 227–239, 2009 AQUATIC MICROBIAL ECOLOGY Printed September 2009 doi: 10.3354/ame01357 Aquat Microb Ecol Published online August 27, 2009 Contribution to AME Special 2 ‘Progress and perspectives in aquatic primary productivity’ OPEN ACCESS Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba David J. Suggett1,*, Noga Stambler2, Ondrej Prá$il3, Zbigniew Kolber4, Antonietta Quigg5, Evaristo Vázquez-Domínguez6, Tamar Zohary7, Tom Berman7, David Iluz8, Orly Levitan2, Tracy Lawson1, Efrat Meeder9,10, Boaz Lazar9,10, Edo Bar-Zeev2, Hana Medova3, Ilana Berman-Frank8 1Department of Biological Sciences, University of Essex, Colchester CO4 3SQ, UK 2Department of Geography and Environment, Bar-Ilan University, Ramat-Gan 52900, Israel 3Photosynthesis Laboratory, Institute of Microbiology ASCR, Opatovicky mlyn, 379 81 Trˇ ebonˇ, Czech Republic 4Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 5Department of Marine Biology and Oceanography, Texas A&M University, Galveston, Texas 77551, USA 6CSIC, Institut de Ciències del Mar, Passeig Marítim de la Barceloneta 39-43, 08003 Barcelona, Spain 7Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research, PO Box 447, Migdal 14950, Israel 8The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel 9The Interuniversity Institute for Marine Sciences Coral Beach, PO Box 469, 88103 Eilat, Israel 10Institute of Earth Sciences, The Hebrew University Edmond J. Safra Campus, Jerusalem 91904, Israel ABSTRACT: Bioassay experiments were performed to identify how growth of key groups within the mi- crobial community was simultaneously limited by nutrient (nitrogen and phosphorus) availability during spring in the Gulf of Aqaba’s oceanic waters. -

Assessing the Chemistry and Bioavailability of Dissolved Organic Matter from Glaciers and Rock Glaciers
RESEARCH ARTICLE Assessing the Chemistry and Bioavailability of Dissolved 10.1029/2018JG004874 Organic Matter From Glaciers and Rock Glaciers Special Section: Timothy Fegel1,2 , Claudia M. Boot1,3 , Corey D. Broeckling4, Jill S. Baron1,5 , Biogeochemistry of Natural 1,6 Organic Matter and Ed K. Hall 1Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO, USA, 2Rocky Mountain Research 3 Key Points: Station, U.S. Forest Service, Fort Collins, CO, USA, Department of Chemistry, Colorado State University, Fort Collins, • Both glaciers and rock glaciers CO, USA, 4Proteomics and Metabolomics Facility, Colorado State University, Fort Collins, CO, USA, 5U.S. Geological supply highly bioavailable sources of Survey, Reston, VA, USA, 6Department of Ecosystem Science and Sustainability, Colorado State University, Fort Collins, organic matter to alpine headwaters CO, USA in Colorado • Bioavailability of organic matter released from glaciers is greater than that of rock glaciers in the Rocky Abstract As glaciers thaw in response to warming, they release dissolved organic matter (DOM) to Mountains alpine lakes and streams. The United States contains an abundance of both alpine glaciers and rock • ‐ The use of GC MS for ecosystem glaciers. Differences in DOM composition and bioavailability between glacier types, like rock and ice metabolomics represents a novel approach for examining complex glaciers, remain undefined. To assess differences in glacier and rock glacier DOM we evaluated organic matter pools bioavailability and molecular composition of DOM from four alpine catchments each with a glacier and a rock glacier at their headwaters. We assessed bioavailability of DOM by incubating each DOM source Supporting Information: with a common microbial community and evaluated chemical characteristics of DOM before and after • Supporting Information S1 • Data Set S1 incubation using untargeted gas chromatography–mass spectrometry‐based metabolomics. -

Interactions Between Photosynthesis and Respiration in an Aquatic Ecosystem
Interactions between Photosynthesis and Respiration in an Aquatic Ecosystem Jane E. Caldwell and Kristi Teagarden 53 Campus Drive, P.O. Box 6057 Dept. of Biology West Virginia University Morgantown, WV 26506 [email protected] (304)293-5201 extension 31459 [email protected] (304)293-5201 extension 31542 Abstract: Students measure the results of respiration and photosynthesis separately, combined, and in comparison to a non-living control “ecosystem”. The living ecosystem uses only snails and water plants. Oxygen, carbon dioxide, and ammonia nitrogen concentrations are measured with simple colorimetric and titration water tests using commercially available kits. The exercise is designed for large enrollment non-majors labs, but modifications for large and small classrooms are described. Introduction This lab exercise was developed for a freshman course for non-science majors at West Virginia University. The exercise asks students to apply their knowledge of basic metabolic processes to a series of simple aquatic ecosystems, which students monitor through water testing. These ecosystems consist of aquaria containing plants and/or snails with or without light exposure, and are compared against a non-living control system (an aquarium with water, light, and gravel). As they analyze their results, students observe the interplay of respiration, photosynthesis, protein digestion (or waste excretion), and decomposition through their effects on dissolved oxygen, carbon dioxide, and ammonia. Students synthesize these observations into written explanations of their results. During the course of the lab, students: • predict the relative levels of oxygen, carbon dioxide, and ammonia for various aquaria compared to a control aquarium. • observe and conduct titrimetric and colorimetric tests for dissolved compounds in water.