Assessing the Chemistry and Bioavailability of Dissolved Organic Matter from Glaciers and Rock Glaciers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbial Community Composition and Function Beneath Temperate Trees Exposed to Elevated Atmospheric Carbon Dioxide and Ozone
Oecologia (2002) 131:236–244 DOI 10.1007/s00442-002-0868-x ECOSYSTEMS ECOLOGY Rebecca L. Phillips · Donald R. Zak William E. Holmes · David C. White Microbial community composition and function beneath temperate trees exposed to elevated atmospheric carbon dioxide and ozone Received: 1 August 2001 / Accepted: 13 December 2001 / Published online: 14 February 2002 © Springer-Verlag 2002 Abstract We hypothesized that changes in plant growth activity and microbial metabolism of cellobiose, and that resulting from atmospheric CO2 and O3 enrichment microbial processes under early-successional aspen and would alter the flow of C through soil food webs and birch species were more strongly affected by CO2 and O3 that this effect would vary with tree species. To test this enrichment than those under late-successional maple. idea, we traced the course of C through the soil microbial community using soils from the free-air CO2 and O3 Keywords Soil microorganisms · enrichment site in Rhinelander, Wisconsin. We added Carbon-13-phospholipid fatty acid analysis · either 13C-labeled cellobiose or 13C-labeled N-acetylglu- Elevated carbon dioxide · Elevated ozone · cosamine to soils collected beneath ecologically distinct Soil carbon cycling temperate trees exposed for 3 years to factorial CO2 –1 (ambient and 200 µl l above ambient) and O3 (ambient and 20 µl l–1 above ambient) treatments. For both labeled Introduction substrates, recovery of 13C in microbial respiration increased beneath plants grown under elevated CO2 by Human activity has increased the concentration of CO2 29% compared to ambient; elevated O3 eliminated this and O3 in the earth’s troposphere (Barnola et al. -

On the Formation of Higher Carbon Oxides in Extreme Environments
Chemical Physics Letters 465 (2008) 1–9 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett FRONTIERS ARTICLE On the formation of higher carbon oxides in extreme environments Ralf I. Kaiser a,*, Alexander M. Mebel b a Department of Chemistry, University of Hawaii at Manoa, 2545 The Mall (Bil 301A), Honolulu, HI 96822, USA b Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA article info abstract Article history: Due to the importance of higher carbon oxides of the general formula COx (x > 2) in atmospheric chem- Received 15 May 2008 istry, isotopic enrichment processes, low-temperature ices in the interstellar medium and in the outer In final form 22 July 2008 solar system, as well as potential implications to high-energy materials, an overview on higher carbon Available online 29 July 2008 oxides COx (x = 3–6) is presented. This article reviews recent developments on these transient species. Future challenges and directions of this research field are highlighted. Ó 2008 Elsevier B.V. All rights reserved. 1. Introduction tion has no activation energy, proceeds with almost unit efficiency, and most likely involves a reaction intermediate. However, neither In recent years, the interest in carbon oxides of higher complex- reaction products (kinetics studies) nor the nature of the interme- 1 + ityP than carbon monoxide (CO; X R ) and carbon dioxide (CO2; diate (kinetics and dynamicsP studies) were determined. On the 1 þ 1 + 3 À X g ) of the generic formula COx (x > 2) has been fueled by com- other hand, a CO(X R )+O2(X g ) exit channel was found to have plex reaction mechanisms of carbon oxides with atomic oxygen in an activation energy between 15 and 28 kJ molÀ1 in the range of the atmospheres of Mars [1–4] and Venus [5]. -
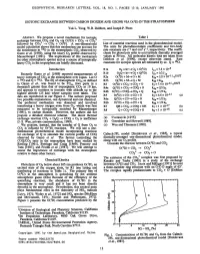
Isotopic Exchange Between Carbon Dioxide and Ozone Via O1D in The
GEOPHYSICAL RESEARCH LETTERS, VOL. 18, NO. 1, PAGES 13-16, JANUARY 1991 ISOTOPICEXCHANGE BETWEEN CARBONDIOXIDE AND OZONEVIA O(!D) IN THE STRATOSPHERE Yuk L. Yung, W.B. DoMore,and Joseph P. Pinto Abstract. We proposea novelmechanism for isotopic Table 1 exchangebetween CO 2 and0 3 vi•aO(ID) + CO2 -->CO_•* followedby CO3' --> CO2 + O(aP). A one-•ensior•al List of essentialreactions used in the photochemicalmodel. model calculation shows that this mechanism can account for The units for photodissociationcoefficients and two-body theenrichment in 180 in thestratospheric CO2 observed by rateconstants are s -1 andcm 3 s-l, respectively.The coeffi- Gatnoeta!. [1989],using the heavy 0 3 profile-observed by cientsfor photolysisrefer to nfid-latitudedimally averaged Mauersberger[1981]. The implicationsof this mechanism values at 30 kan. All molecular kinetic data are taken frmn for otherstratospheric species and as a sourceof isotopically DoMore etal. [1990], except otherwise stated. Rate heavyCO 2 in thetroposphere are briefly discussed. constantsforisotopic species are estimated byus. Q = 180. h•troduction Rla O3+ bY-->O2 + O(1D) Jla=7.4x10 -5 RecentlyGamo et at. [1989] reportedmeasurements of Rib O2Q+ hv-->O2 + Q(!D) Jlb=1/2 J la heavyisotopes of CO2 in the stratosphereover Japan. Let O R2a O(ID)+ M--->O+ M k2a= 2.0x 10-11 e ll0/T = 160 andQ = 180.The fiQ of stratosphericCO2, as defined R2b Q(1D) + M --->Q + M k2b= k2a in Gatno et at., was found to be 2O/oo(two parts in a R3 O(ID)+ CO2---->CO2 + O k3 = 7.4x 10-11 e 120/T thousand)greater than that of troposphericCO 2 at 19 km, R4a Q(1D)+ CO2-->CO Q + O k4a= 2/3k 3 and appearsto continueto increasewith altitudeup to the R4b O(ID)+ COQ-->CO2 + Q k4b= 1/3k 3 highestaltitude (25 km) where sampleswere taken. -

A Two-Step Graph Convolutional Decoder for Molecule Generation
A Two-Step Graph Convolutional Decoder for Molecule Generation Xavier Bresson Thomas Laurent School of Computer Science and Engineering Department of Mathematics NTU, Singapore Loyola Marymount University [email protected] [email protected] Abstract We propose a simple auto-encoder framework for molecule generation. The molec- ular graph is first encoded into a continuous latent representation z, which is then decoded back to a molecule. The encoding process is easy, but the decoding pro- cess remains challenging. In this work, we introduce a simple two-step decoding process. In a first step, a fully connected neural network uses the latent vector z to produce a molecular formula, for example CO2 (one carbon and two oxygen atoms). In a second step, a graph convolutional neural network uses the same latent vector z to place bonds between the atoms that were produced in the first step (for example a double bond will be placed between the carbon and each of the oxygens). This two-step process, in which a bag of atoms is first generated, and then assembled, provides a simple framework that allows us to develop an efficient molecule auto-encoder. Numerical experiments on basic tasks such as novelty, uniqueness, validity and optimized chemical property for the 250k ZINC molecules demonstrate the performances of the proposed system. Particularly, we achieve the highest reconstruction rate of 90.5%, improving the previous rate of 76.7%. We also report the best property improvement results when optimization is constrained by the molecular distance between the original and generated molecules. 1 Introduction A fundamental problem in drug discovery and material science is to design molecules with arbitrary optimized chemical properties. -

Potential Ivvs.Gelbasedre
US010644304B2 ( 12 ) United States Patent ( 10 ) Patent No.: US 10,644,304 B2 Ein - Eli et al. (45 ) Date of Patent : May 5 , 2020 (54 ) METHOD FOR PASSIVE METAL (58 ) Field of Classification Search ACTIVATION AND USES THEREOF ??? C25D 5/54 ; C25D 3/665 ; C25D 5/34 ; ( 71) Applicant: Technion Research & Development HO1M 4/134 ; HOTM 4/366 ; HO1M 4/628 ; Foundation Limited , Haifa ( IL ) (Continued ) ( 72 ) Inventors : Yair Ein - Eli , Haifa ( IL ) ; Danny (56 ) References Cited Gelman , Haifa ( IL ) ; Boris Shvartsev , Haifa ( IL ) ; Alexander Kraytsberg , U.S. PATENT DOCUMENTS Yokneam ( IL ) 3,635,765 A 1/1972 Greenberg 3,650,834 A 3/1972 Buzzelli ( 73 ) Assignee : Technion Research & Development Foundation Limited , Haifa ( IL ) (Continued ) ( * ) Notice : Subject to any disclaimer , the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 CN 1408031 4/2003 U.S.C. 154 ( b ) by 56 days . EP 1983078 10/2008 (21 ) Appl. No .: 15 /300,359 ( Continued ) ( 22 ) PCT Filed : Mar. 31 , 2015 OTHER PUBLICATIONS (86 ) PCT No .: PCT/ IL2015 /050350 Hagiwara et al. in ( Acidic 1 - ethyl - 3 -methylimidazoliuum fluoride: a new room temperature ionic liquid in Journal of Fluorine Chem $ 371 (c ) ( 1 ), istry vol . 99 ( 1999 ) p . 1-3 ; ( Year: 1999 ). * (2 ) Date : Sep. 29 , 2016 (Continued ) (87 ) PCT Pub . No .: WO2015 / 151099 Primary Examiner — Jonathan G Jelsma PCT Pub . Date : Oct. 8 , 2015 Assistant Examiner Omar M Kekia (65 ) Prior Publication Data (57 ) ABSTRACT US 2017/0179464 A1 Jun . 22 , 2017 Disclosed is a method for activating a surface of metals , Related U.S. Application Data such as self- passivated metals , and of metal -oxide dissolu tion , effected using a fluoroanion -containing composition . -

1 Andrews Glacier in August 2016 Tyndall Glacier Lidar Scan in May
Final Report for “Glacier and perennial snowfield mass balance of Rocky Mountain National Park (ROMO): Past, Present, and Future” Task Agreement Number P16AC00826 PI Dr. Daniel McGrath June 28, 2019 Department of Geosciences Colorado State University [email protected] Andrews Glacier in August 2016 Tyndall Glacier LiDAR scan in May 2016 1 Summary of Key Project Outcomes • Over the past ~50 years, geodetic glacier mass balances for four glaciers along the Front Range have been highly variable; for example, Tyndall Glacier thickened slightly, while Arapaho Glacier thinned by >20 m. These glaciers are closely located in space (~30 km) and hence the regional climate forcing is comparable. This variability points to the important role of local topographic/climatological controls (such as wind-blown snow redistribution and topographic shading) on the mass balance of these very small glaciers (~0.1-0.2 km2). • Since 2001, glacier area (for 11 glaciers on the Front Range) has varied ± 40%, with changes most commonly driven by interannual variability in seasonal snow. However, between 2001 and 2017, the glaciers exhibited limited net change in area. Previous work (Hoffman et al., 2007) found that glacier area had started to decline starting in ~2000. • Seasonal mass turnover is very high for Andrews and Tyndall glaciers. On average, the glaciers gain and lose ~9 m of elevation each year. Such extraordinary amounts of snow accumulation is primarily the result of wind-blown snow redistribution into these basins (and to a certain degree, avalanching at Tyndall Glacier) and exceeds observed peak snow water equivalent at a nearby SNOTEL station by 5.5 times. -

Microbial Metabolism, Chemistry, and Communities Under Study at the UCLA-DOE Institute for Genomics and Proteomics Rachel R
Microbial Metabolism, Chemistry, and Communities under Study at the UCLA-DOE Institute for Genomics and Proteomics Rachel R. Ogorzalek Loo* ([email protected]), John Muroski, Brendan Mahoney, Orlando Martinez, Janine Fu, Joseph A. Loo, Robert Clubb, Robert Gunsalus, and Todd Yeates 1UCLA-DOE Institute for Genomics and Proteomics, Los Angeles, CA https://www.doe-mbi.ucla.edu/ Project Goals: Research in the UCLA-DOE Institute for Genomics and Proteomics includes major efforts to elucidate critical microbial processes that decompose and recycle plant, animal and microbial biomass. Towards this end, we seek to decipher the metabolism of syntrophic microbial communities and examine how anaerobic microbes assemble complex cellulosome structures that degrade lignocellulose. Biomass decomposition and recycling occur in essentially all anaerobic habitats on Earth, as well as in industrial/municipal waste treatment applications. Unfortunately, the current understanding of these critical processes is insufficient to enable modeling and prediction of environmental carbon flow. The benefits from increasing our knowledge of anaerobic decomposition include optimizing the attack and release of plant wall-derived molecules destined for biofuel and industrial feedstock production and improving biogas/sewage and waste stream processing plant design and operation. Our DOE sponsored research seeks to advance the understanding of syntrophic-based microbial metabolism at molecular- and systems-levels and its role in biomass recycling/remediation. Exploring the pathways and key enzyme reactions of syntrophy begins by mining the genomes of previously unstudied syntrophic bacteria, such as those that metabolize model aliphatic fatty acid and amino acid substrates. That only minimal experimental data pertaining to these organisms is available severely limits the ability to draw conclusions from genome sequence alone, and even adding transcriptomic data may not suffice. -

Microbial Lithification in Marine Stromatolites and Hypersaline Mats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Published in Trends in Microbiology 13,9 : 429-438, 2005, 1 which should be used for any reference to this work Microbial lithification in marine stromatolites and hypersaline mats Christophe Dupraz1 and Pieter T. Visscher2 1Institut de Ge´ ologie, Universite´ de Neuchaˆ tel, Rue Emile-Argand 11, CP 2, CH-2007 Neuchaˆ tel, Switzerland 2Center for Integrative Geosciences, Department of Marine Sciences, University of Connecticut, 1080 Shennecossett Road, Groton, Connecticut, 06340, USA Lithification in microbial ecosystems occurs when pre- crucial role in regulating sedimentation and global bio- cipitation of minerals outweighs dissolution. Although geochemical cycles. the formation of various minerals can result from After the decline of stromatolites in the late Proterozoic microbial metabolism, carbonate precipitation is pos- (ca. 543 million years before present), microbially induced sibly the most important process that impacts global and/or controlled precipitation continued throughout the carbon cycling. Recent investigations have produced geological record as an active and essential player in most models for stromatolite formation in open marine aquatic ecosystems [9,10]. Although less abundant than in environments and lithification in shallow hypersaline the Precambrian, microbial precipitation is observed in a lakes, which could be highly relevant for interpreting the multitude of semi-confined (physically or chemically) -

Fegel Colostate 0053N 13488.Pdf (1.978Mb)
THESIS BETWEEN A ROCK AND A HARD PLACE; THE CHEMISTRY, BIOLOGY, AND LABILITY OF GLACIAL MELTWATERS IN THE AMERICAN WEST Submitted by Timothy Scott Fegel II Graduate Degree Program in Ecology In partial fulfillment of the requirements For the Degree of Master of Science Colorado State University Fort Collins, Colorado Spring 2016 Master’s Committee: Advisor: Jill Baron Co-advisor: Edward Hall Michael Gooseff Copyright by Timothy Scott Fegel 2016 All Rights Reserved ABSTRACT BETWEEN A ROCK AND A HARD PLACE; THE CHEMISTRY, BIOLOGY, AND LABILITY OF GLACIAL MELTWATERS IN THE AMERICAN WEST Glaciers and rock glaciers supply water and nutrients to headwater mountain lakes and streams across all regions of the American West. The resulting changes in volume, timing, and chemistry of meltwater discharged by these features appears to be having significant effects on the adjacent alpine headwater ecosystems they feed. Whereas both glaciers and rock glaciers are sources of seasonal meltwater, sediment, and solutes to headwater ecosystems, differences in meltwater characteristics between glacial types, and its affect on biological productivity, is poorly documented. Here we present a comparative study of the metal, nutrient, and microbial characteristics of glacial and rock glacial influence on headwater ecosystems in three mountain ranges of the contiguous U.S.: the Cascade Mountains, Rocky Mountains, and Sierra Nevada. Several meltwater characteristics (water temperature, conductivity, pH, heavy metals, nutrients, complexity of dissolved organic matter (DOM), and bacterial richness and diversity) differed significantly between glacier and rock glacier 2+ 3+ meltwaters, while other characteristics (Ca , Fe , SiO2 concentrations, reactive nitrogen, and microbial processing of DOM) showed distinct charcteristics between mountain ranges regardless of meltwater source. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Module 3 Microbial Metabolism
Module 3 Microbial Metabolism © 2013 Pearson Education, Inc. Chapter 5 Microbial Metabolism © 2013 Pearson Education, Inc. Lectures prepared by Helmut Kae Lectures prepared by Christine L. Case Copyright © 2013 Pearson Education, Inc. Catabolic and Anabolic Reactions § Metabolism: the sum of the chemical reactions in an organism § Two general types of metabolic reactions § Catabolism: provides energy and building blocks for anabolism § Anabolism: uses energy and building blocks to build large molecules § Recall from Chapter 2 § Energy can be stored when covalent bonds form § Energy can be released when covalent bonds broken © 2013 Pearson Education, Inc. Catabolic Reactions § Breakdown of complex substances to small molecules § Degradative reactions § Breaking of covalent bonds § What type of reaction used to break covalent bonds? § Purpose is to generate energy, make building blocks § Releases energy stored in bonds § Breakdown products used to build new molecules § Eg, breakdown of glucose for energy © 2013 Pearson Education, Inc. Anabolic Reactions § Building macromolecules from small molecules § Biosynthetic reactions § Forming of covalent bonds § What type of reaction used to form covalent bonds? § Requires energy to form bonds § Purpose is to generate materials for cell growth § Eg, making proteins from amino acids © 2013 Pearson Education, Inc. Figure 5.1 The role of ATP in coupling anabolic and catabolic reactions. Catabolism releases energy by oxidation of molecules Energy is Energy is released by stored in hydrolysis molecules of ATP of ATP Anabolism uses energy to synthesize macromolecules that make up the cell © 2013 Pearson Education, Inc. Catabolic and Anabolic Reactions § A metabolic pathway is a sequence of chemical reactions in a cell § Metabolic pathways are controlled by enzymes © 2013 Pearson Education, Inc. -

Primary Productivity Below the Seafloor at Deep-Sea Hot Springs
Primary productivity below the seafloor at deep-sea hot springs Jesse McNichola,1,2, Hryhoriy Stryhanyukb, Sean P. Sylvac, François Thomasa,3, Niculina Musatb, Jeffrey S. Seewaldc, and Stefan M. Sieverta,1 aBiology Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543; bDepartment of Isotope Biogeochemistry, Helmholtz Centre for Environmental Research – Umweltforschungszentrum (UFZ), 04318 Leipzig, Germany; and cMarine Chemistry and Geochemistry Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543 Edited by David M. Karl, University of Hawaii, Honolulu, HI, and approved May 16, 2018 (received for review March 13, 2018) Below the seafloor at deep-sea hot springs, mixing of geothermal but only one such measurement has been previously obtained under fluids with seawater supports a potentially vast microbial ecosys- realistic temperature and pressure conditions (4). Another im- tem. Although the identity of subseafloor microorganisms is largely portant consideration is that electron acceptors such as oxygen and known, their effect on deep-ocean biogeochemical cycles cannot nitrate rapidly become limiting during incubation experiments with be predicted without quantitative measurements of their meta- vent fluids (13, 14), which may lead to carbon fixation rates being bolic rates and growth efficiency. Here, we report on incubations greatly underestimated (13). However, it is difficult to ascertain the of subseafloor fluids under in situ conditions that quantitatively extent of this bias for existing studies because electron acceptor constrain subseafloor primary productivity, biomass standing stock, consumption has not typically been measured alongside carbon and turnover time. Single-cell-based activity measurements and 16S fixation. Theoretical estimates of primary productivity have also rRNA-gene analysis showed that Campylobacteria dominated car- been derived by combining geochemical measurements with ther- bon fixation and that oxygen concentration and temperature drove modynamic models (15, 16).