Fegel Colostate 0053N 13488.Pdf (1.978Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glacial Surface Area Change in Grand Teton National Park Jake Edmunds
Glacial Surface Area change in Grand Teton National Park Jake Edmunds Meriden, Wyoming Glenn Tootle Civil and Architectural Enginnering _________________________________ Introduction: The Intergovernmental Panel on Climatic Change (IPCC) reported that a consensus exists among scientists and policy makers that “…the globally averaged net effect of human activities since 1750 has been one of warming…” (IPCC, 2007). The objective of the proposed research is to investigate glacial change in Grand Teton National Park (GTNP). Remote sensing data was obtained for the GTNP and a preliminary analysis of glacier area change was performed. Since the glaciated regions of GTNP have not been intensely studied in the past, it is essential to understand the past behaviors of the glaciers in the region. This study aims to create a database of quantitative information for the glaciers in GTNP such that future observations may be compared to past observations in an attempt to identify any long term trends of glacier behavior. The research aimed to document glacial surface area change for selected glaciers in the Teton Mountain Range via analysis of aerial photographs (preliminary analysis). Aerial photographs were obtained from the USGS Earth Resources Observation & Science (EROS) Data Center in Sioux Falls, South Dakota. Each image will be analyzed with a photogrammetric (the process of obtaining quantitative information from photographs) approach. The proposed approach involves digitizing and georeferencing each photo using ArcGIS. Once the georeferencing process is performed each glacier can be delineated using an unsupervised classification. Areas of snow and ice tend to have distinct reflectance values in aerial photographs, thus those areas can be delineated using an unsupervised classification. -

Jellyfish Invasion Researchers Study the Toxins, Tentacles and DNA of New Jersey’S Newest Annoyance INSIGHTS | COLLEGE of SCIENCE and MATHEMATICS
FALL 2017 WHERE DISCOVERY AND INNOVATION MEET VOLUME 3, NUMBER 2 THE RESEARCH CHRONICLE OF THE COLLEGE OF SCIENCE AND MATHEMATICS insights Jellyfish Invasion Researchers study the toxins, tentacles and DNA of New Jersey’s newest annoyance INSIGHTS | COLLEGE OF SCIENCE AND MATHEMATICS FROM THE DEAN contents VOLUME 3, NUMBER 2 I FALL 2017 The Creative Scientist Stereotypes of scientists abound – but rarely does the description of “a creative type” conjure up the image of a scientist. A creative person has originality of thought and expression, and is open to transcending traditional ideas, rules and patterns to create meaningful new ideas or interpretations. Of Jellyfish course, scientists are creative! We work to put the pieces together, to ask the right questions and perform experiments that lead to more questions. Astronomer Carl Sagan once noted that, “It is the tension 6 between creativity and skepticism that has produced the stunning and unexpected findings of science.” Invasion It is indeed these unexpected findings that nudge science forward, sometimes in small increments and Researchers study the toxins, sometimes in major “eureka” leaps. In fact, modern science has become so large and so complex that tentacles and DNA of New many of the biggest challenges require scientific teams that extend their creativity by combining fields of Jersey’s newest annoyance expertise and approaches in new ways. College of Science and Mathematics (CSAM) scholars embrace the call to be creative and keep their minds open to the unexpected, unusual or unpredicted result. In this issue of Insights, scientists, Microbes to the Rescue mathematicians and educators explore important questions and answers, adding to the body of scientific Researchers harness healthy soil for the “greening” of 2 brownfields. -

To See the Hike Archive
Geographical Area Destination Trailhead Difficulty Distance El. Gain Dest'n Elev. Comments Allenspark 932 Trail Near Allenspark A 4 800 8580 Allenspark Miller Rock Riverside Dr/Hwy 7 TH A 6 700 8656 Allenspark Taylor and Big John Taylor Rd B 7 2300 9100 Peaks Allenspark House Rock Cabin Creek Rd A 6.6 1550 9613 Allenspark Meadow Mtn St Vrain Mtn TH C 7.4 3142 11632 Allenspark St Vrain Mtn St Vrain Mtn TH C 9.6 3672 12162 Big Thompson Canyon Sullivan Gulch Trail W of Waltonia Rd on Hwy A 2 941 8950 34 Big Thompson Canyon 34 Stone Mountain Round Mtn. TH B 8 2100 7900 Big Thompson Canyon 34 Mt Olympus Hwy 34 B 1.4 1438 8808 Big Thompson Canyon 34 Round (Sheep) Round Mtn. TH B 9 3106 8400 Mountain Big Thompson Canyon Hwy 34 Foothills Nature Trail Round Mtn TH EZ 2 413 6240 to CCC Shelter Bobcat Ridge Mahoney Park/Ginny Bobcat Ridge TH B 10 1500 7083 and DR trails Bobcat Ridge Bobcat Ridge High Bobcat Ridge TH B 9 2000 7000 Point Bobcat Ridge Ginny Trail to Valley Bobcat Ridge TH B 9 1604 7087 Loop Bobcat Ridge Ginny Trail via Bobcat Ridge TH B 9 1528 7090 Powerline Tr Boulder Chautauqua Park Royal Arch Chautauqua Trailhead by B 3.4 1358 7033 Rgr. Stn. Boulder County Open Space Mesa Trail NCAR Parking Area B 7 1600 6465 Boulder County Open Space Gregory Canyon Loop Gregory Canyon Rd TH B 3.4 1368 7327 Trail Boulder Open Space Heart Lake CR 149 to East Portal TH B 9 2000 9491 Boulder Open Space South Boulder Peak Boulder S. -
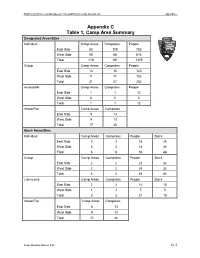
Appendix C Table 1, Camp Area Summary
Backcountry/Wilderness Management Plan and Environmental Assessment Appendix C Appendix C Table 1, Camp Area Summary Designated Areas/Sites Individual Camp Areas Campsites People East Side 60 109 763 West Side 59 88 616 Total 119 197 1379 Group Camp Areas Campsites People East Side 10 10 120 West Side 11 11 132 Total 21 21 252 Accessible Camp Areas Campsites People East Side 1 1 12 West Side 0 0 0 Total 1 1 12 Wood Fire Camp Areas Campsites East Side 8 13 West Side 9 13 Total 17 26 Stock Areas/Sites Individual Camp Areas Campsites People Stock East Side 3 3 18 24 West Side 3 3 18 24 Total 6 6 36 48 Group Camp Areas Campsites People Stock East Side 2 2 24 32 West Side 2 2 24 32 Total 4 4 48 64 Llama only Camp Areas Campsites People Stock East Side 2 2 14 10 West Side1175 Total 3 3 21 15 Wood Fire Camp Areas Campsites East Side 8 13 West Side 9 13 Total 17 26 Rocky Mountain National Park C-1 Backcountry/Wilderness Management Plan and Environmental Assessment Appendix C Crosscountry Areas Areas Parties People East Side 9 16 112 West Side 14 32 224 Total 23 48 336 Summer Totals for Designated, Stock and Crosscountry Areas Camp Areas Campsites/Parties People East Side 80 136 1004 West Side 84 131 969 Total 164 267 1973 Bivouac Areas Areas People East Side 11 88 West Side 0 0 Total 11 88 Winter Areas Areas Parties People East Side 32 136 1632 West Side 23 71 852 Total 55 207 2484 Rocky Mountain National Park C-2 Backcountry/Wilderness Management Plan and Environmental Assessment Appendix C Appendix C Table 2, Designated Camp Area/Sites Number -

Assessing the Chemistry and Bioavailability of Dissolved Organic Matter from Glaciers and Rock Glaciers
RESEARCH ARTICLE Assessing the Chemistry and Bioavailability of Dissolved 10.1029/2018JG004874 Organic Matter From Glaciers and Rock Glaciers Special Section: Timothy Fegel1,2 , Claudia M. Boot1,3 , Corey D. Broeckling4, Jill S. Baron1,5 , Biogeochemistry of Natural 1,6 Organic Matter and Ed K. Hall 1Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO, USA, 2Rocky Mountain Research 3 Key Points: Station, U.S. Forest Service, Fort Collins, CO, USA, Department of Chemistry, Colorado State University, Fort Collins, • Both glaciers and rock glaciers CO, USA, 4Proteomics and Metabolomics Facility, Colorado State University, Fort Collins, CO, USA, 5U.S. Geological supply highly bioavailable sources of Survey, Reston, VA, USA, 6Department of Ecosystem Science and Sustainability, Colorado State University, Fort Collins, organic matter to alpine headwaters CO, USA in Colorado • Bioavailability of organic matter released from glaciers is greater than that of rock glaciers in the Rocky Abstract As glaciers thaw in response to warming, they release dissolved organic matter (DOM) to Mountains alpine lakes and streams. The United States contains an abundance of both alpine glaciers and rock • ‐ The use of GC MS for ecosystem glaciers. Differences in DOM composition and bioavailability between glacier types, like rock and ice metabolomics represents a novel approach for examining complex glaciers, remain undefined. To assess differences in glacier and rock glacier DOM we evaluated organic matter pools bioavailability and molecular composition of DOM from four alpine catchments each with a glacier and a rock glacier at their headwaters. We assessed bioavailability of DOM by incubating each DOM source Supporting Information: with a common microbial community and evaluated chemical characteristics of DOM before and after • Supporting Information S1 • Data Set S1 incubation using untargeted gas chromatography–mass spectrometry‐based metabolomics. -

1 Andrews Glacier in August 2016 Tyndall Glacier Lidar Scan in May
Final Report for “Glacier and perennial snowfield mass balance of Rocky Mountain National Park (ROMO): Past, Present, and Future” Task Agreement Number P16AC00826 PI Dr. Daniel McGrath June 28, 2019 Department of Geosciences Colorado State University [email protected] Andrews Glacier in August 2016 Tyndall Glacier LiDAR scan in May 2016 1 Summary of Key Project Outcomes • Over the past ~50 years, geodetic glacier mass balances for four glaciers along the Front Range have been highly variable; for example, Tyndall Glacier thickened slightly, while Arapaho Glacier thinned by >20 m. These glaciers are closely located in space (~30 km) and hence the regional climate forcing is comparable. This variability points to the important role of local topographic/climatological controls (such as wind-blown snow redistribution and topographic shading) on the mass balance of these very small glaciers (~0.1-0.2 km2). • Since 2001, glacier area (for 11 glaciers on the Front Range) has varied ± 40%, with changes most commonly driven by interannual variability in seasonal snow. However, between 2001 and 2017, the glaciers exhibited limited net change in area. Previous work (Hoffman et al., 2007) found that glacier area had started to decline starting in ~2000. • Seasonal mass turnover is very high for Andrews and Tyndall glaciers. On average, the glaciers gain and lose ~9 m of elevation each year. Such extraordinary amounts of snow accumulation is primarily the result of wind-blown snow redistribution into these basins (and to a certain degree, avalanching at Tyndall Glacier) and exceeds observed peak snow water equivalent at a nearby SNOTEL station by 5.5 times. -

A Guide to the Geology of Rocky Mountain National Park, Colorado
A Guide to the Geology of ROCKY MOUNTAIN NATIONAL PARK COLORADO For sale by the Superintendent of Documents, Washington, D. C. Price 15 cents A Guide to the Geology of ROCKY MOUNTAIN NATIONAL PARK [ COLORADO ] By Carroll H. Wegemann Former Regional Geologist, National Park Service UNITED STATES DEPARTMENT OF THE INTERIOR HAROLD L. ICKES, Secretary NATIONAL PARK SERVICE . NEWTON B. DRURY, Director UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1944 Table of Contents PAGE INTRODUCTION in BASIC FACTS ON GEOLOGY 1 THE OLDEST ROCKS OF THE PARK 2 THE FIRST MOUNTAINS 3 The Destruction of the First Mountains 3 NATURE OF PALEOZOIC DEPOSITS INDICATES PRESENCE OF SECOND MOUNTAINS 4 THE ROCKY MOUNTAINS 4 Time and Form of the Mountain Folding 5 Erosion Followed by Regional Uplift 5 Evidences of Intermittent Uplift 8 THE GREAT ICE AGE 10 Continental Glaciers 11 Valley Glaciers 11 POINTS OF INTEREST ALONG PARK ROADS 15 ROAD LOGS 18 Thompson River Entrance to Deer Ridge Junction 18 Deer Ridge Junction to Fall River Pass via Fall River .... 20 Fall River Pass to Poudre Lakes 23 Trail Ridge Road between Fall River Pass and Deer Ridge Junction 24 Deer Ridge Junction to Fall River Entrance via Horseshoe Park 29 Bear Lake Road 29 ILLUSTRATIONS LONGS PEAK FROM BEAR LAKE Front and back covers CHASM FALLS Inside back cover FIGURE PAGE 1. GEOLOGIC TIME SCALE iv 2. LONGS PEAK FROM THE EAST 3 3. PROFILE SECTION ACROSS THE ROCKY MOUNTAINS 5 4. ANCIENT EROSIONAL PLAIN ON TRAIL RIDGE 6 5. ANCIENT EROSIONAL PLAIN FROM FLATTOP MOUNTAIN ... 7 6. VIEW NORTHWEST FROM LONGS PEAK 8 7. -

Mercury and Other Trace Elements in Glacial Meltwater at Grand Te 2
Carling et al.: Mercury and Other Trace Elements in Glacial Meltwater at Grand Te 2 MERCURY AND OTHER TRACE ELEMENTS IN GLACIAL MELTWATER AT GRAND TETON NATIONAL PARK, WYOMING GREGORY T. CARLING DAVID G. TINGEY BRIGHAM YOUNG UNIVERSITY DIEGO P. FERNANDEZ UNIVERSITY OF UTAH ABSTRACT Hg methylation in the proglacial streams. Other trace elements were found in low concentrations in the melt Glaciers are a reservoir of mercury (Hg) and water, but increased substantially downstream of the other trace elements that have accumulated in the ice glaciers due to water-rock interactions. during the industrial era. As glaciers continue to melt at an alarming rate, these potentially toxic metals are released to the environment. In order to evaluate the INTRODUCTION impact of glacier melt on water quality in high elevation catchments in Grand Teton National Park, The retreat of glaciers worldwide may lead to we sampled transects along the Teton and Middle the rapid release of mercury and other trace metals to Teton glaciers and proglacial streams during early- high elevation aquatic ecosystems (Barbante et al. July and mid-August 2013. The glaciers were snow- 2004, Hong et al. 2004, Schuster et al. 2002). Mercury covered during July, and thus water samples were is a toxic element that is deposited to aquatic systems primarily melt of snowpack from the previous winter. primarily by atmospheric deposition. Due to enhanced The glacier ice was exposed during August, and thus orographic-driven wet and dry deposition at high samples likely represented true glacier melt. These elevations, glaciers likely receive disproportionately contrasting sample sets allowed for a determination of high mercury loads (Carling et al. -

Rocky Mountain National Park Geologic Resource Evaluation Report
National Park Service U.S. Department of the Interior Geologic Resources Division Denver, Colorado Rocky Mountain National Park Geologic Resource Evaluation Report Rocky Mountain National Park Geologic Resource Evaluation Geologic Resources Division Denver, Colorado U.S. Department of the Interior Washington, DC Table of Contents Executive Summary ...................................................................................................... 1 Dedication and Acknowledgements............................................................................ 2 Introduction ................................................................................................................... 3 Purpose of the Geologic Resource Evaluation Program ............................................................................................3 Geologic Setting .........................................................................................................................................................3 Geologic Issues............................................................................................................. 5 Alpine Environments...................................................................................................................................................5 Flooding......................................................................................................................................................................5 Hydrogeology .............................................................................................................................................................6 -

VITAL SIGNS 2014 Science and Resource Managment Grand Teton National Park & John D
Science and Resource Management National Park Service Grand Teton National Park U.S. Department of the Interior & John D. Rockefeller, Jr. Memorial Parkway GRAND TETON NATIONAL PARK & John D. Rockefeller, Jr. Memorial Parkway Natural and Cultural Resources VITAL SIGNS 2014 Science and Resource Managment Grand Teton National Park & John D. Rockefeller, Jr. Memorial Parkway P.O. Drawer 170 Moose, WY 83012 www.nps.gov/grte 2 Vital Signs 2014 • Grand Teton National Park Acknowledgments Special thanks to the Grand Teton Association whose generous support made production of this report possible. To supplement the work done by the Grand Teton Resource Management staf, the following organizations provided data and/or analysis that were used in preparing this report: Biodiversity Research Institute Craighead Beringia South Colorado State University, Federal Land Manager Environmental Database Grand Teton Fire Management Program Grand Teton Jenny Lake Rangers Greater Yellowstone Inventory and Monitoring Network Greater Yellowstone Whitebark Pine Monitoring Working Group Interagency Grizzly Bear Study Team (U.S. Geological Survey–Biological Resources Division, National Park Service, U.S. Forest Service, and the states of Idaho, Montana, and Wyoming) National Park Service Air Resources Division National Park Service Northern Rockies Exotic Plant Management Team Sky Aviation U.S. Fish and Wildlife Service, National Elk Refuge U.S. Forest Service, Bridger Teton National Forest U.S. Geological Survey, Northern Rocky Mountain Science Center -

Contributions to the Geography of the United States, 1922
CONTRIBUTIONS TO THE GEOGRAPHY OF THE UNITED STATES, 1922. PENEPLAINS OF4 THE FRONT RANGE AND ROCKY MOUNTAIN NATIONAL PARK, COLORADO. By Wnxis T. LEE. PURPOSE OF THE PAPER. The purpose of this paper is to call attention to some of the major surface features in the Rocky Mountain National Park and to point out their probable correlation with similar features in neigh boring regions. The observations on which the paper is based were made in the summer of 1916, during an investigation in which other work demanded first consideration. This paper may therefore be considered a by-product. For the same reason many of the observa tions were not followed to conclusions, yet the data obtained seem to be sufficient to establish a certain order of events, the recognition of which may be of assistance in working out in detail the geologic and geographic history of the Rocky Mountain region. TWO CYCLES OF EROSION PREVIOUSLY RECOGNIZED. In an account of the general geology of the Georgetown quad rangle, Colo.j Ball x describes three distinct types of land forms " an old, mature mountainous upland; younger V-shaped valleys in cised in this upland; and glacial cirques developed at the heads of some of the streams, passing downward into U-shaped valleys." Ball describes the mountainous upland as consisting of highlands of rounded or gently sloping tops which are remnants of an ancient peneplain or old surface of erosion. Most of these remnants in the Georgetown quadrangle lie above an altitude of 11,500 feet. On thig old peneplain there were many monadnocks, some of which are now recognizable as the summits of the highest mountains. -

Microbial Assemblages Reflect Environmental Heterogeneity in Alpine Streams
Received: 8 November 2018 | Revised: 1 April 2019 | Accepted: 26 April 2019 DOI: 10.1111/gcb.14683 PRIMARY RESEARCH ARTICLE Microbial assemblages reflect environmental heterogeneity in alpine streams Scott Hotaling1 | Mary E. Foley1,2 | Lydia H. Zeglin3 | Debra S. Finn4 | Lusha M. Tronstad5 | J. Joseph Giersch6 | Clint C. Muhlfeld6,7 | David W. Weisrock1 1Department of Biology, University of Kentucky, Lexington, Kentucky Abstract 2Biology Department, Rutgers, The State Alpine streams are dynamic habitats harboring substantial biodiversity across small University of New Jersey, Camden, New spatial extents. The diversity of alpine stream biota is largely reflective of environ‐ Jersey 3Division of Biology, Kansas State mental heterogeneity stemming from varying hydrological sources. Globally, alpine University, Manhattan, Kansas stream diversity is under threat as meltwater sources recede and stream conditions 4 Department of Biology, Missouri State become increasingly homogeneous. Much attention has been devoted to macroin‐ University, Springfield, Missouri vertebrate diversity in alpine headwaters, yet to fully understand the breadth of cli‐ 5Wyoming Natural Diversity Database, University of Wyoming, Laramie, mate change threats, a more thorough accounting of microbial diversity is needed. Wyoming We characterized microbial diversity (specifically Bacteria and Archaea) of 13 6U.S. Geological Survey, Northern Rocky Mountain Science Center, West Glacier, streams in two disjunct Rocky Mountain subranges through 16S rRNA gene sequenc‐ Montana ing. Our study encompassed the spectrum of alpine stream sources (glaciers, snow‐ 7 Flathead Lake Biological Station, The fields, subterranean ice, and groundwater) and three microhabitats (ice, biofilms, and University of Montana, Polson, Montana streamwater). We observed no difference in regional (γ) diversity between subranges Correspondence but substantial differences in diversity among ( ) stream types and microhabitats.