Fermentation and Anaerobic Decomposition in a Hot Spring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
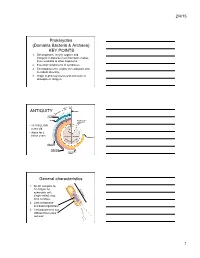
Prokaryotes (Domains Bacteria & Archaea)
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

Terrestrial Decomposition
Terrestrial Decomposition • Objectives – Controls over decomposition • Litter breakdown • Soil organic matter formation and dynamics – Carbon balance of ecosystems • Soil carbon storage 1 Overview • In terrestrial ecosystems, soils (organic horizon + mineral soil) > C than in vegetation and atmosphere combined 2 Overview • Decomposition is: 1. Major pathway for C loss from ecosystems 2. Central to ecosystem C loss and storage 3 Overview 4 Incorporation 1 year later Overview • Predominant controls on litter decomposition are fairly well constrained 1. Temperature and moisture 2. Litter quality • N availability • Lignin concentration • Lignin:N • Mechanisms for soil organic matter stabilization: 1. Recalcitrance (refers to chemistry) 2. Physical protection • Within soil aggregates • Organo-mineral associations 3. Substrate supply regulation (energetic limitation) 5 Overview • Disturbance can override millenia in a matter of days or years: 1. Land use change 2. Invasive species 3. Climate change • Understanding the mechanistic drivers of decomposition, soil organic matter formation, and carbon stabilization help us make management decisions, take mitigation steps, and protect resources. 6 Overview Native Ōhiʻa - Koa forest Conversion to Reforestation in grass-dominated pasture (80 yr) Eucalyptus plantation (10 yr) Conventional sugar cane harvest. 20° C 18° C 16° C 14° C 7 Sustainable ratoon harvest. Decomposition • Decomposition is the biological, physical and chemical breakdown of organic material – Provides energy for microbial growth -

Aerobic Respiration
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: + - C6H12O6 + 6 H2O ==> 6 CO2 + 24 H +24 e oxidation electron donor (aka energy source) + - (O2+ 4H + 4e ==> 2H2O) x6 reduction electron acceptor --------------------------------------- C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. - • Aerobic respiration: e acceptor is O2 - • Anaerobic respiration: e acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? carbon source? organic organic CO CO compound 2 compound 2 chemoheterotroph chemoautotroph photoheterotroph photoautotroph e- acceptor? Nitrifying and sulfur- use H O to reduce CO ? oxidizing bacteria 2 2 green non-sulfur and O Other than O 2 2 purple non-sulfur bacteria anoxygenic oxygenic photosynthesis: photosynthesis: green sulfur and most bacteria Organic Inorganic cyanobacteria compound compound purple sulfur bacteria fermentative organism anaerobic respiration: nitrate, sulfate, Fe(III) Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the -

Nitrogen and Phosphorus Limitation of Oceanic Microbial Growth During Spring in the Gulf of Aqaba
Vol. 56: 227–239, 2009 AQUATIC MICROBIAL ECOLOGY Printed September 2009 doi: 10.3354/ame01357 Aquat Microb Ecol Published online August 27, 2009 Contribution to AME Special 2 ‘Progress and perspectives in aquatic primary productivity’ OPEN ACCESS Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba David J. Suggett1,*, Noga Stambler2, Ondrej Prá$il3, Zbigniew Kolber4, Antonietta Quigg5, Evaristo Vázquez-Domínguez6, Tamar Zohary7, Tom Berman7, David Iluz8, Orly Levitan2, Tracy Lawson1, Efrat Meeder9,10, Boaz Lazar9,10, Edo Bar-Zeev2, Hana Medova3, Ilana Berman-Frank8 1Department of Biological Sciences, University of Essex, Colchester CO4 3SQ, UK 2Department of Geography and Environment, Bar-Ilan University, Ramat-Gan 52900, Israel 3Photosynthesis Laboratory, Institute of Microbiology ASCR, Opatovicky mlyn, 379 81 Trˇ ebonˇ, Czech Republic 4Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 5Department of Marine Biology and Oceanography, Texas A&M University, Galveston, Texas 77551, USA 6CSIC, Institut de Ciències del Mar, Passeig Marítim de la Barceloneta 39-43, 08003 Barcelona, Spain 7Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research, PO Box 447, Migdal 14950, Israel 8The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel 9The Interuniversity Institute for Marine Sciences Coral Beach, PO Box 469, 88103 Eilat, Israel 10Institute of Earth Sciences, The Hebrew University Edmond J. Safra Campus, Jerusalem 91904, Israel ABSTRACT: Bioassay experiments were performed to identify how growth of key groups within the mi- crobial community was simultaneously limited by nutrient (nitrogen and phosphorus) availability during spring in the Gulf of Aqaba’s oceanic waters. -

Plant Species Effects on Nutrient Cycling: Revisiting Litter Feedbacks
Review Plant species effects on nutrient cycling: revisiting litter feedbacks Sarah E. Hobbie Department of Ecology, Evolution and Behavior, University of Minnesota, St. Paul, MN 55108, USA In a review published over two decades ago I asserted reinforce those gradients and patterns of NPP, focusing that, along soil fertility gradients, plant traits change in on feedbacks operating through plant litter decomposition. ways that reinforce patterns of soil fertility and net Specifically, I evaluate two key assumptions underlying primary productivity (NPP). I reevaluate this assertion the plant litter feedback idea: (i) plant litter traits vary in light of recent research, focusing on feedbacks to NPP predictably along fertility gradients, and (ii) such variation operating through litter decomposition. I conclude that reinforces soil fertility gradients through effects on decom- mechanisms emerging since my previous review might position and litter N release. Given the number of synthetic weaken these positive feedbacks, such as negative cross-site analyses of plant traits and their consequences effects of nitrogen on decomposition, while others for nutrient cycling over the past two decades, the time is might strengthen them, such as slower decomposition ripe for revisiting my original assertions. Indeed, I show of roots compared to leaf litter. I further conclude that that my original assertion is more nuanced and complex predictive understanding of plant species effects on than originally claimed. In particular, I discuss the need to nutrient cycling will require developing new frameworks consider leaf litter decomposition more carefully and move that are broadened beyond litter decomposition to con- beyond consideration of leaf litter feedbacks to a more sider the full litter–soil organic matter (SOM) continuum. -

Organic Matter Decomposition in Simulated Aquaculture Ponds Group Fish Culture and Fisheries Daily Supervisor(S) Dr
O rganic matter decomposition in simulated aquaculture ponds Beatriz Torres Beristain Promotor: Prof. Dr. J.A .J. V erreth H oogleraar in de V isteelt en V isserij W ageningen U niversiteit C o-promotor: Dr. M .C .J. V erdegem U niversitair docent bij the Leerstoelgroep V isteelt en V isserij W ageningen U niversiteit Samenstelling promotiecommissie: Prof. Dr. Y . A vnimelech Technion, Israel Institute of Technology Prof. Dr. Ir. H .J. Gijzen U N ESC O -IH E, Delf, N etherlands Prof. Dr. Ir. M . W .A . V erstegen W ageningen U niversiteit Prof. Dr. Ir. A .A . K oelmans W ageningen U niversiteit Dit onderzoek is uitgevoerd binnen de onderzoekschool W IA S O rganic matter decomposition in simulated aquaculture ponds Beatriz Torres Beristain Proefschrift Ter verkrijging van de graad van doctor O p gezag van de rector magnificus van W ageningen U niversiteit, Prof. Dr. Ir. L. Speelman, In het openbaar te verdedigen O p dinsdag 15 A pril 2005 des namiddags te half tw ee in de A ula Torres Beristain, B. O rganic matter decomposition in simulated aquaculture ponds PhD thesis, Fish C ulture and Fisheries Group, W ageningen Institute of A nimal Sciences. W ageningen U niversity, P.O . Box 338, 6700 A H W ageningen, The N etherlands. - W ith R ef. œW ith summary in Spanish, Dutch and English ISBN : 90-8504-170-8 A Domingo, Y olanda y A lejandro Table of contents C hapter 1 General introduction. 1 C hapter 2 R eview microbial ecology and role in aquaculture ponds. -

Exploring the Nutritional Values of Hydrogenotrophic Bacteria As Space Food
ISSN: 2455-2631 © December 2018 IJSDR | Volume 3, Issue 12 Exploring the Nutritional Values of Hydrogenotrophic Bacteria as Space Food 1M.GIRISH, 2S.ABIRAMI, 3Dr.V.MAHALAKSHMI 1,2Research scholar, 3Professor 1,3Madras Christian College, 2V.R.R Institute of Biomedical Sciences. Abstract: Hydrogenotrophs are organisms that are capable of metabolizing molecular hydrogen as source of energy and hence hydrogenotrophy is performed by carbon dioxide reducing organism to produce methane. Methanogens are diverse group of strict anaerobes where methanogenesis is a major route in the anaerobic mineralization of organic matter. They have very unique enzyme and cofactors involved in this metabolic pathway. The anaerobic chamber provides a convenient culture system for large-scale studies of strictly anaerobic and/or facultative anaerobic bacteria. In this study, the Hydrogenotrophic organism isolated was characterized as Methanosarcina sp. Though these hydrogenotrophs utilise carbon dioxide and grow in a minimal growth requirement, they were found to be rich in carbohydrates and protein content. Therefore, Hydrogenotroph being a good food source, can be used for future space travel where the astronauts could carry them aboard and synthesis their own food. The by-products produced by these organisms can be used to produce a protein rich meal. This system can be incorporated on to space craft where this works as a closed loop between astronauts and hydrogenotrophs, where carbon dioxide released by astronauts can be captured and filtered through a system and supplied for the growth of these microbes which in turn synthesis carbohydrates. Keywords: Hydrogenotrophs, space food, anaerobes. I. INTRODUCTION Hydrogenotrophs are organisms that are capable of metabolizing molecular hydrogen as source of energy. -

Principles of Ecology Section ●2 Flow of Energy in an Ecosystem
chapter 2 Principles of Ecology section ●2 Flow of Energy in an Ecosystem -!). )DEA Before You Read Autotrophs capture energy, making it available for all If a pet had to survive without your care, how would its diet members of a food web. change? Write your ideas on the lines below. Read about how What You’ll Learn organisms get food and energy in their environment. ■ the fl ow of energy through an ecosystem ■ food chains, food webs, and pyramid models 3TUDY#OACH Read to Learn Make Flash Cards Make a fl ash card for each question Energy in an Ecosystem heading in this section. On the One way to study the interactions within an ecosystem is to back of the fl ash card, write the trace how energy fl ows through the system. All organisms are answer to the question. Use the classifi ed by the way they obtain energy. fl ash cards to review what you have learned. How do autotrophs obtain energy? All green plants and other organisms that produce their own food are the primary producers of food in an ecosystem. They are called autotrophs. An autotroph (AW tuh trohf) is an organism that captures energy from sunlight or inorganic substances to produce food. Autotrophs make energy available for all other organisms in the ecosystem. How do heterotrophs differ from autotrophs? A heterotroph (HE tuh roh trohf), also called a consumer, is an organism that obtains energy by consuming other organisms. A heterotroph that consumes only plants is an herbivore (HUR buh vor). Cows, rabbits, and grasshoppers are herbivores. -

Decomposition Responses to Climate Depend on Microbial Community Composition
Decomposition responses to climate depend on microbial community composition Sydney I. Glassmana,b,1, Claudia Weihea, Junhui Lic, Michaeline B. N. Albrighta,d, Caitlin I. Loobya,e, Adam C. Martinya,c, Kathleen K. Tresedera, Steven D. Allisona, and Jennifer B. H. Martinya aDepartment of Ecology and Evolutionary Biology, University of California, Irvine, CA 92697; bDepartment of Microbiology and Plant Pathology, University of California, Riverside, CA 92521; cDepartment of Earth System Science, University of California, Irvine, CA 92697; dBioscience Division, Los Alamos National Laboratory, Los Alamos, NM 87545; and eDepartment of Biological Sciences, University of Denver, CO 80210 Edited by Mary K. Firestone, University of California, Berkeley, CA, and approved October 10, 2018 (received for review June 29, 2018) Bacteria and fungi drive decomposition, a fundamental process in soil moisture and extracellular enzyme production across a natural the carbon cycle, yet the importance of microbial community climate gradient (16). composition for decomposition remains elusive. Here, we used an Given that the factors regulating decomposition are often 18-month reciprocal transplant experiment along a climate gradient context dependent and can vary in their influence across a range in Southern California to disentangle the effects of the microbial of spatial and temporal scales (17, 18), we hypothesized that community versus the environment on decomposition. Specifically, decomposition responses to changing climatic conditions would we tested whether the decomposition response to climate change depend on microbial community composition. To test this hy- depends on the microbial community. We inoculated microbial pothesis, we conducted the largest microbial community trans- decomposers from each site onto a common, irradiated leaf litter plant experiment to date. -

Organic Matter Decomposition and Ecosystem Metabolism As Tools to Assess the Functional Integrity of Streams and Rivers–A Systematic Review
water Review Organic Matter Decomposition and Ecosystem Metabolism as Tools to Assess the Functional Integrity of Streams and Rivers–A Systematic Review Verónica Ferreira 1,* , Arturo Elosegi 2 , Scott D. Tiegs 3 , Daniel von Schiller 4 and Roger Young 5 1 Marine and Environmental Sciences Centre–MARE, Department of Life Sciences, University of Coimbra, 3000–456 Coimbra, Portugal 2 Faculty of Science and Technology, University of the Basque Country (UPV/EHU), P.O. Box 644, 48080 Bilbao, Spain; [email protected] 3 Department of Biological Sciences, Oakland University, Rochester, MI 48309, USA; [email protected] 4 Department of Evolutionary Biology, Ecology and Environmental Sciences, Institut de Recerca de l’Aigua (IdRA), University of Barcelona, Diagonal 643, 08028 Barcelona, Spain; [email protected] 5 Cawthron Institute, Private Bag 2, Nelson 7042, New Zealand; [email protected] * Correspondence: [email protected] Received: 4 November 2020; Accepted: 8 December 2020; Published: 15 December 2020 Abstract: Streams and rivers provide important services to humans, and therefore, their ecological integrity should be a societal goal. Although ecological integrity encompasses structural and functional integrity, stream bioassessment rarely considers ecosystem functioning. Organic matter decomposition and ecosystem metabolism are prime candidate indicators of stream functional integrity, and here we review each of these functions, the methods used for their determination, and their strengths and limitations for bioassessment. We also provide a systematic review of studies that have addressed organic matter decomposition (88 studies) and ecosystem metabolism (50 studies) for stream bioassessment since the year 2000. Most studies were conducted in temperate regions. Bioassessment based on organic matter decomposition mostly used leaf litter in coarse-mesh bags, but fine-mesh bags were also common, and cotton strips and wood were frequent in New Zealand.