The Preparation of Iodic Acid and Its A~~Yd~I~~~~ by Arthur B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office Patented Apr
3,378,337 United States Patent Office Patented Apr. 16, 1968 1. 2 and the hydrogen peroxide. This result is achieved without 3,378,337 contaminating the reaction mixture. The final solution PREPARATHON OF ODEC ACID AND DERVATIVES THEREOF contains substantially pure iodic acid, and can be evap Ricardo O. Bach, Gastonia, N.C., assignor to Lithium orated and dehydrated to obtain the iodic acid, or the Corporation of America, Inc., New York, N.Y., a cor anhydride thereof, or it can be used as a medium for the poration of Minnesota direct production of salts of iodic acid. No Drawing. Fied May 17, 1965, Ser. No. 456,561 In carrying out the method of the present invention, 12 Claims. (CI. 23-85) the iodic acid solution employed in forming the reaction mixture should contain sufficient iodic acid to provide a O hydrogen ion and iodate ion concentration in the reaction ABSTRACT OF THE DISCLOSURE mixture which will favor oxidation of iodine to its penta A method of preparing solutions of substantially pure valent state and impede decomposition of hydrogen iodic acid from which salts of the acid, the anhydride peroxide. Generally speaking, the quantity of iodic acid thereof and/or the crystalline form of the acid can be present in the starting solution should not be below about 5 0.5%, with especially good results being attained with directly obtained. The method involves reacting iodine from about 1% to about 10%, usually about 5%, of the with hydrogen peroxide in the presence of a relatively quantity of iodic acid to be produced. -
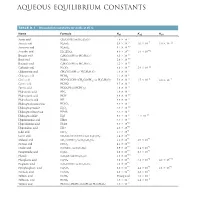
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

Stencil Remover 285 Safety Data Sheet 01 June 2015
Safety Data Sheet Section 1: Identification of the Substance/Mixture and of the Company/Undertaking 1.1 Product identifier Product Name • Stencil Remover 285 Product Description • Clear liquid. 1.2 Relevant identified uses of the substance or mixture and uses advised against Relevant identified use(s) • Liquid stencil remover concentrate 1.3 Details of the supplier of the safety data sheet Manufacturer • IKONICS Corporation 4832 Grand Ave. Duluth, MN 55807 United States www.ikonics.com [email protected] Telephone (General) • (218) 628-2217 Telephone (General) • (800) 328-4261 - Toll free 1.4 Emergency telephone number Chemtrec • 1-800-424-9300 - Within USA and Canada • +1 703-527-3887 - Outside USA and Canada (collect calls accepted) Section 2: Hazards Identification EU/EEC According to EU Directive 1272/2008 (CLP)/REACH 1907/2006 [amended by 453/2010] According to EU Directive 67/548/EEC (DSD) or 1999/45/EC (DPD) 2.1 Classification of the substance or mixture CLP • Oxidizing Liquids 2 - H272 Skin Corrosion 1 - H314 Serious Eye Damage 1 - H318 DSD/DPD • Corrosive (C) R35 2.2 Label Elements CLP DANGER Hazard statements • H272 - May intensify fire; oxidizer H314 - Causes severe skin burns and eye damage Stencil Remover 285 Safety Data Sheet Page 1 of 12 H318 - Causes serious eye damage Precautionary statements Prevention • P210 - Keep away from heat. P220 - Keep/Store away from clothing and other combustible materials. P221 - Take any precaution to avoid mixing with combustibles P260 - Do not breathe dusts or mists. P264 - Wash thoroughly after handling. P280 - Wear protective gloves/protective clothing/eye protection/face protection. -
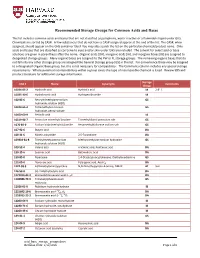
Common Acid and Base Storage Group Recommendations
Recommended Storage Groups for Common Acids and Bases This list includes common acids and bases that are not classified as pyrophoric, water reactive or a flammable liquid under GHS. Chemicals are sorted by CAS#. A few substances that do not have a CAS# assigned appear at the end of the list. The CAS#, when assigned, should appear on the GHS container label. You may also search the list on the particular chemical/product name. Only acids and bases that are classified as corrosive to eyes and/or skin under GHS are included. The solvent for select acid or base solutions are given in parenthesis after the name. Organic acids [OA], inorganic acids [IA], and inorganic bases [IB] are assigned to designated storage groups. Many organic bases are assigned to the PW or FL storage groups. The remaining organic bases that do not fall into any other storage group are assigned the General Storage group [GS] in this list. For convenience these may be assigned to a designated Organic Base group, but this is not necessary for compatibility. The Comments column includes any special storage requirements. Where potential incompatibilities within a group exists the type of incompatible chemical is listed. Review SDS and product literature for additional storage information. Storage CAS # Name Synonyms Comments Group 10034-85-2 Hydriodic acid Hydriotic acid IA 2-8° C 10035-10-6 Hydrobromic acid Hydrogen Bromide IA 100-85-6 Benzyltrimethylammonium GS hydroxide solution (H2O) 10424-65-4 Tetramethylammonium GS hydroxide, pentahydrate 10450-60-9 Periodic acid -

The Rate of The: Reaction Between Iodic and Hydriodic Acids
THE RATE OF THE: REACTION BETWEEN IODIC AND HYDRIODIC ACIDS BY SAUL DUSHMAN In 1888 the rate of reaction between iodic and hydriodic acids was made the subject of a research by 0. Burchard,I who endeavored to discover a relation between the rate and the con- centrations of these two reagents. His results, however, were unsatisfactory, and the author concludes his paper with the words, ((Inspite of all attempts, I have not succeeded in represent- ing the rate of the reaction by a general differential equation ”. In light of the electrolytic dissociation theory introduced by Arrhenius just at the time of Burchard’s publication, it now seetns advisable to represent hydrogen iodide in solution as con- + - sisting almost entirely of hydrion (H) and iodion (I); simi- + larly a solution of iodic acid contains hydrion (H) and iodation - - (IO3), while brown “iodine ” solution contains tri-iodion (I3). Thus, in place of Burchard’s equation, 5HI f- HIO, = 31, + 3H,O, (1) it seems better to write + -- - 6H + 81 + IO, = 31, + 3H,O, (2) and in consequence, I have studied the effect of the concentra- T-- - tions of H, I, IO3 and I3 on the rate, instead of adopting the variables chosen by Burchard. In planning the experiments, I adopted a method which was originally employ-ed by Harcourt and Esson in their classi- cal research, “On the Observation of the Course of Chemical Change”, in 1866,‘ but which had, apparently been completely forgotten by 1888. The principle of the method is very sim- Zeit. phys. Chem. 2, 796 (1888). Jour. -
Acid Nomenclature.Notebook February 02, 2017 Writing Acid Formulas/Naming Acids Formula Starts with "H" NameEnds in "Acid"
Acid nomenclature.notebook February 02, 2017 Writing Acid Formulas/Naming acids formula starts with "H" nameends in "acid" Balance the overall charge to zero... (like ionic criss cross) +1 3 All acids start with H+1 H PO4 H3PO4 Identify the acid: cation is always H+ use the anion to name it 3 ways to name: 1. binary acids ("bi" = "2") or nonoxy acid H+ and one other element anion ends in ide HCl Cl1 chloride hydro_____ic acid HF F1 fluoride hydro_____ic acid H2S S2 sulfide hydro_____ic acid 3 H3N N nitride hydro_____ic acid Binary rule: hydrostemic acid use stem from anion 2. Oxyacid acids contain oxygen Again, look at the anion 1 HNO3 NO3 nitrate _____ic acid 2 _______ic acid H2SO4 SO4 sulfate 3 _______ic acid H3PO4 PO4 phosphate oxyacid rule #1: Oxyacid rule #1: if anion ends in ate, use stemic acid I ate something icky in the cafeteria 1 HNO2 NO2 nitrite _____ous acid 2 _______ous acid H2SO3 SO3 sulfite 3 _______ous acid H3PO3 PO3 phosphite oxyacid rule #2: if anion ends in ite, use stemous acid 1 Acid nomenclature.notebook February 02, 2017 acid nomenclature: (review) Acids binary (nonoxy) oxyacid ______ide ______ite ______ate hydro______ic acid _______ous acid _______ic acid 2 Acid nomenclature.notebook February 02, 2017 Naming Acids Anion Ending Acid Name Binary @ ide hydro(stem)ic acid ate (stem) ic acid Oxyacid/ Ternary ite (stem) ous acid An easy way to remember which goes with which… “In the cafeteria, you ATE something ICky” 3 Acid nomenclature.notebook February 02, 2017 Naming Acids -
Measurement of Iodine Species and Sulfuric Acid Using Bromide
https://doi.org/10.5194/amt-2020-494 Preprint. Discussion started: 18 December 2020 c Author(s) 2020. CC BY 4.0 License. 1 Measurement of iodine species and sulfuric acid using bromide 2 chemical ionization mass spectrometers 3 4 Mingyi Wang1,2*, Xu-Cheng He3*#, Henning Finkenzeller4, Siddharth Iyer3, Dexian Chen1,5, Jiali 5 Shen3, Mario Simon6, Victoria Hofbauer1,2, Jasper Kirkby6,7, Joachim Curtius6, Norbert Maier8, 6 Theo Kurtén3,8, Douglas R. Worsnop3,9, Markku Kulmala3,10,11,12, Matti Rissanen3,13, Rainer 7 Volkamer4, Yee Jun Tham3#, Neil M. Donahue1,2,5,14, and Mikko Sipilä3 8 9 1Center for Atmospheric Particle Studies, Carnegie Mellon University, Pittsburgh, PA, 15213, USA 10 2Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA, 15213, USA 11 3Institute for Atmospheric and Earth System Research (INAR), University of Helsinki, 00014 Helsinki, Finland 12 4Department of Chemistry & CIRES, University of Colorado Boulder, Boulder, CO 80309, USA 13 5Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, PA, 15213, USA 14 6Institute for Atmospheric and Environmental Sciences, Goethe University Frankfurt, 60438 Frankfurt am Main, 15 Germany 16 7CERN, the European Organization for Nuclear Research, CH-1211 Geneve 23, Switzerland 17 8Department of Chemistry, University of Helsinki, 00014 Helsinki, Finland 18 9Aerodyne Research, Inc., Billerica, MA, 01821, USA 19 10Helsinki Institute of Physics, P.O. BoX 64 (Gustaf Hallstromin katu 2), FI-00014 University of Helsinki, Finland 20 11Joint International Research -

Binary Acids, Oxyacids, Bases, Hydrates
Working backwards: name to formula Naming Compounds • It’s possible to determine a formula from a name • E.g. What is the formula of sodium oxide? • To get the answer, first write the valences: 1 2 Na O → Na2O • What is the formula of copper(II) oxide? 2 2 Cu O → Cu2O2 → CuO • For covalent compounds, simply use the prefixes to tell you the number of each element: • What is the formula for dinitrogen trioxide? N2O3 • Give formulae for: lithium sulfide, dinitrogen Day 2 monoxide, lead(IV) sulfate Assignment Naming Bases 1. Name each according to IUPAC rules: • Bases contain an OH group •CH O does not have an OH group a) ZnS, b) FeCl3, c) CaCO3, d) P2O5, e) NaCN, 6 12 6 • If an OH group is present it will be clearly f) N2F2, g) MgHPO4, h) Cu(BrO3)2, i) K2O, j) BF3 2. Give the valence of a) Fe in FeO, b) Mn in MnO indicated: e.g. NaOH, Ca(OH)2 2 • Also notice that bases have a metal (or positive 3. Write formulas for: a) sodium oxide, ion such as NH + at their beginning) b) potassium iodide, c) plumbic sulfide, 4 • Bases are named like other ionic compounds: d) mercury(I) oxide, e) ferrous oxide, • +ve is named first, followed by the polyatomic ion f) iron(II) phosphate, g) copper(II) fluoride, h) dichlorine monoxide, i) silver sulfide, Ca(OH)2 j) magnesium nitride, k) aluminum hypochlorite, CuOH l) iodine pentafluoride, m) calcium chromate, aluminum hydroxide n) diphosphorus pentasulfide ammonium hydroxide Naming Acids: Binary acids Naming Acids: Oxyacids • Naming does not depend on the state (aq) • All acids start with H (e.g. -

Selective Benzylic C–H Monooxygenation Mediated by Iodine Oxides
Selective benzylic C–H monooxygenation mediated by iodine oxides Kelsey B. LaMartina1, Haley K. Kuck1, Linda S. Oglesbee1, Asma Al-Odaini1 and Nicholas C. Boaz*1,2,3 Full Research Paper Open Access Address: Beilstein J. Org. Chem. 2019, 15, 602–609. 1Department of Chemistry and Physics, North Central College, 30 N. doi:10.3762/bjoc.15.55 Brainard Street, Naperville, IL 60540 USA, 2Department of Chemistry, Frick Chemical Laboratory, Princeton University, Washington Road, Received: 13 December 2018 Princeton, NJ 08544 USA and 3Permanent address: Department of Accepted: 19 February 2019 Chemistry, North Central College, 30 N. Brainard Street, Naperville, IL Published: 05 March 2019 60540 USA; phone: +1-630-637-5187. Associate Editor: C. Stephenson Email: Nicholas C. Boaz* - [email protected] © 2019 LaMartina et al.; licensee Beilstein-Institut. License and terms: see end of document. * Corresponding author Keywords: acetoxylation; benzylic; iodate; NHPI; oxidation; radical Abstract A method for the selective monooxdiation of secondary benzylic C–H bonds is described using an N-oxyl catalyst and a hyperva- lent iodine species as a terminal oxidant. Combinations of ammonium iodate and catalytic N-hydroxyphthalimide (NHPI) were shown to be effective in the selective oxidation of n-butylbenzene directly to 1-phenylbutyl acetate in high yield (86%). This method shows moderate substrate tolerance in the oxygenation of substrates containing secondary benzylic C–H bonds, yielding the corresponding benzylic acetates in good to moderate yield. Tertiary benzylic C–H bonds were shown to be unreactive under similar conditions, despite the weaker C–H bond. A preliminary mechanistic analysis suggests that this NHPI-iodate system is functioning by a radical-based mechanism where iodine generated in situ captures formed benzylic radicals. -

Dissociation Constants of Inorganic Acids and Bases
DISSOCIATION CONSTANTS OF INORGANIC ACIDS AND BASES The data in this table are presented as values of pKa, defined as can be calculated from the equation the negative logarithm of the acid dissociation constant K for the a − + reaction OH NH4 = = KKb water/K a []NH BH B– + H+ 3 + where K = 1.01 10–14 at 25 °C. Note that pK + pK = pK . Thus pKa = –log Ka , and the hydrogen ion concentration [H ] can water × a b water be calculated from All values refer to dilute aqueous solutions at zero ionic strength at the temperature indicated. The table is arranged alphabetically + − HB by compound name. = K a [] BH Reference In the case of bases, the entry in the table is for the conjugate acid; – 1. Perrin, D. D., Ionization Constants of Inorganic Acids and Bases in e.g., ammonium ion for ammonia. The OH concentration in the Aqueous Solution, Second Edition, Pergamon, Oxford, 1982. system + – NH3 + H2O NH4 + OH Name Formula Step t/°C pKa Aluminum(III) ion Al+3 25 5.0 Ammonia NH3 25 9.25 Arsenic acid H3AsO4 1 25 2.26 2 25 6.76 3 25 11.29 Arsenious acid H2AsO3 25 9.29 Barium(II) ion Ba+2 25 13.4 Boric acid H3BO3 1 20 9.27 2 20 >14 Calcium(II) ion Ca+2 25 12.6 Carbonic acid H2CO3 1 25 6.35 2 25 10.33 Chlorous acid HClO2 25 1.94 Chromic acid H2CrO4 1 25 0.74 2 25 6.49 Cyanic acid HCNO 25 3.46 Germanic acid H2GeO3 1 25 9.01 2 25 12.3 Hydrazine N2H4 25 8.1 Hydrazoic acid HN3 25 4.6 Hydrocyanic acid HCN 25 9.21 Hydrofluoric acid HF 25 3.20 Hydrogen peroxide H2O2 25 11.62 Hydrogen selenide H2Se 1 25 3.89 2 25 11.0 Hydrogen sulfide H2S 1