Oxidation States of Nitrogen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Group 17 (Halogens)
Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2020 Dr. Susan Findlay See Exercises 11.1 to 11.4 Forms of Carbon The Halogens (Group 17) What is a halogen? Any element in Group 17 (the only group containing Cl2 solids, liquids and gases at room temperature) Exists as diatomic molecules ( , , , ) Melting Boiling 2State2 2 2Density Point Point (at 20 °C) (at 20 °C) Fluorine -220 °C -188 °C Gas 0.0017 g/cm3 Chlorine -101 °C -34 °C Gas 0.0032 g/cm3 Br2 Bromine -7.25 °C 58.8 °C Liquid 3.123 g/cm3 Iodine 114 °C 185 °C Solid 4.93 g/cm3 A nonmetal I2 Volatile (evaporates easily) with corrosive fumes Does not occur in nature as a pure element. Electronegative; , and are strong acids; is one of the stronger weak acids 2 The Halogens (Group 17) What is a halogen? Only forms one monoatomic anion (-1) and no free cations Has seven valence electrons (valence electron configuration . ) and a large electron affinity 2 5 A good oxidizing agent (good at gaining electrons so that other elements can be oxidized) First Ionization Electron Affinity Standard Reduction Energy (kJ/mol) Potential (kJ/mol) (V = J/C) Fluorine 1681 328.0 +2.866 Chlorine 1251 349.0 +1.358 Bromine 1140 324.6 +1.065 Iodine 1008 295.2 +0.535 3 The Halogens (Group 17) Fluorine, chlorine and bromine are strong enough oxidizing agents that they can oxidize the oxygen in water! When fluorine is bubbled through water, hydrogen fluoride and oxygen gas are produced. -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Understanding Pigments: the Third Step to Higher Quality And
Understanding Pigments: The Third Mark Harber October, 2000 Step to Higher Quality and Consistency Putting great color in your product is part of the pigments. However, they are less opaque and systems approach for resolving issues of sub- would have to be used at higher loading levels to standard properties and appearance. achieve similar whiteness and opacity. This article on pigments is the third in a four-part Titanium Dioxide is used in the majority of the series about the interrelationship of the material products made by the cast polymer industry. Tita- components used in marble and solid surface nium Dioxide-based colors include most whites, manufacturing. These AOC-authored articles re- pastels, earth tones and off-whites such as bone, spond to the challenge that the cast polymer in- ivory, beige or biscuit. As noted in Table 1, non- dustries aspire to higher standards of quality and white synthetic oxides are combined with Titani- consistency. Because resolving cast polymer is- um Dioxide to create pastels and earth tones for sues requires a systems approach, other articles cultured marble and solid surface applications. in this series address resins, gel coats and pro- cessing. All articles begin with background infor- Phthalocyanine pigments, or "Phthalos," impart mation on the main subject matter, followed by deep colors such as the automotive "Hunter ten related issues and guidelines. Green" of a sport utility vehicle or the high strength Blue used in ballpoint pens. Because A BACKGROUND ON COLORANTS they are so deep when used by themselves, In their natural state, cast polymer resins meet a Phthalo Blue and Phthalo Green are normally variety of performance requirements but are lack- blended with other pigments, many times Titani- ing in the color that draws the customer to the um Dioxide. -

United States Patent Office
2,752,270 United States Patent Office Patented June 26, 1956 2 These impurities impair the crystallization of the solu 2,752,270 tions for the recovery of glucose; on the one hand, they PROCESS OF HYDROLYZNG wooD N PRE. increase the solubility of the glucose in the solvent, and PARNG CRYSTALLINE GLUCOSE on the other hand they retard the growth of the glucose Hugo Specht, Mannheim-Rheinau, Germany, assignor 5 crystals which remain so snail as to be very difficult to to Deutsche Bergin-Aktiengesellschaft, Mannheim separate from the syrup. I have found that crystalline Rheinau, Germany glucose can be obtained in good yields only when the No Drawing. Application January 5, 1954, Wood sugar Solution subjected to crystallization is so Serial No. 402,401 prepared as to consist essentially of glucose and to be free Claims priority, application Germany January 31, 1949 0 as far as possible from the recited impurities, including mannose. According to the invention, cellulosic ma 4 Claims. (C. 127-37) terial, for instance wood, is essentially freed from hemi The invention relates to improvements in the prepara celluloses; the residue should contain not more than tion of crystalline glucose by the hydrolysis of wood and about 5-6 per cent of sugars other than glucose, cal is a continuation-in-part of my copending application, 5 culated on the total content of carbohydrates, and is Serial No. 242,172, filed August 16, 1951, now aban then hydrolyzed with superconcentrated (39–42%) hy doned. drochloric acid. The thus obtained strongly acidic sugar Several processes have already been proposed for the solution is freed from hydrochloric acid by evaporation recovery of crystalline glucose from wood sugar solu So as to leave only a small residue of the acid; the mass tions, but they could not be economically operated on a 20 is then diluted to form a liquor containing about 10 to large scale. -

United States Patent Office Patented Apr
3,378,337 United States Patent Office Patented Apr. 16, 1968 1. 2 and the hydrogen peroxide. This result is achieved without 3,378,337 contaminating the reaction mixture. The final solution PREPARATHON OF ODEC ACID AND DERVATIVES THEREOF contains substantially pure iodic acid, and can be evap Ricardo O. Bach, Gastonia, N.C., assignor to Lithium orated and dehydrated to obtain the iodic acid, or the Corporation of America, Inc., New York, N.Y., a cor anhydride thereof, or it can be used as a medium for the poration of Minnesota direct production of salts of iodic acid. No Drawing. Fied May 17, 1965, Ser. No. 456,561 In carrying out the method of the present invention, 12 Claims. (CI. 23-85) the iodic acid solution employed in forming the reaction mixture should contain sufficient iodic acid to provide a O hydrogen ion and iodate ion concentration in the reaction ABSTRACT OF THE DISCLOSURE mixture which will favor oxidation of iodine to its penta A method of preparing solutions of substantially pure valent state and impede decomposition of hydrogen iodic acid from which salts of the acid, the anhydride peroxide. Generally speaking, the quantity of iodic acid thereof and/or the crystalline form of the acid can be present in the starting solution should not be below about 5 0.5%, with especially good results being attained with directly obtained. The method involves reacting iodine from about 1% to about 10%, usually about 5%, of the with hydrogen peroxide in the presence of a relatively quantity of iodic acid to be produced. -

1 the Volumetric Determination of Hydroxylamine
VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. I363 [CONTRIBUTION FROM THE CHEMICAL LABORATORYOF THE UNIVERSITY OF CALIFORNIA.1 THE VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. BY WILLIAMC. BRAY,MIBUM E. SIMPSONAND ANNA A. MACKENZIE. Received July 17, 1919 In the present investigation 3 volumetric methods of determining hydroxylamine in aqueous solution have been studied : The titanous salt method,' in which the hydroxylamine is reduced by excess titanous salt in acid solution with exclusion of air, and the excess titrated with permanganate. 2NH20H + Ti2(S04)3 = (NH4)2S04 + 4TiOS04 + HzS04. (I) The ferric salt method,2 in which the hydroxylamine is oxidized in an acid solution by excess of a ferric salt, the mixture is boiled and the fer- rous salt formed titrated with permanganate. 2NH20H + 2Fe@04)3 = N2O + 4FeS04 + 2H2S04 + H20. (2) The iodine method,3 in which the hydroxylamine is oxidized by iodine in a neutral solution, e. g., in the presence of disodium phosphate. 2NH20H + 212 = N2O + 4HI + H2O (3) or 2NH20H + 213- = N20 + 61- + 4H+ + HzO. Our first experiments, with the iodine method, yielded irregular results which could not be interpreted until the concentration of the hydroxyl- amine solution was accurately determined. An examination of the literature showed a rather unsatisfactory state of affairs. The advocates of the ferric sulfate method furnish evidence that it is perfectly reliable, but Leuba4 gives detailed experimental data to prove the contrary, and Adams5 states that he could not obtain reproducible results with it. The investigators who have used the iodine method consider it to be fairly satisfactory, but some of them state that it is not very accurate, and Rupp and Maeder6 have recently concluded that correct results are obtained only by a compensation of errors. -

Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=13374 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-25481-6 Toxicology; National Research Council 356 pages 6 x 9 PAPERBACK (2012) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. -

Rate Constants for Reactions Between Iodine- and Chlorine-Containing Species: a Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction†
3708 J. Am. Chem. Soc. 1996, 118, 3708-3719 Rate Constants for Reactions between Iodine- and Chlorine-Containing Species: A Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction† Istva´n Lengyel,‡ Jing Li, Kenneth Kustin,* and Irving R. Epstein Contribution from the Department of Chemistry and Center for Complex Systems, Brandeis UniVersity, Waltham, Massachusetts 02254-9110 ReceiVed NoVember 27, 1995X Abstract: The chlorite-iodide reaction is unusual because it is substrate-inhibited and autocatalytic. Because - analytically pure ClO2 ion is not easily prepared, it was generated in situ from the rapid reaction between ClO2 and I-. The resulting overall reaction is multiphasic, consisting of four separable parts. Sequentially, beginning with mixing, these parts are the (a) chlorine dioxide-iodide, (b) chlorine(III)-iodide, (c) chlorine(III)-iodine, and (d) hypoiodous and iodous acid disproportionation reactions. The overall reaction has been studied experimentally and by computer simulation by breaking it down into a set of kinetically active subsystems and three rapidly established - equilibria: protonations of chlorite and HOI and formation of I3 . The subsystems whose kinetics and stoichiometries were experimentally measured, remeasured, or which were previously experimentally measured include oxidation of - iodine(-1,0,+1,+3) by chlorine(0,+1,+3), oxidation of I by HIO2, and disproportionation of HOI and HIO2. The final mechanism and rate constants of the overall reaction and of its subsystems were determined by sensitivity analysis and parameter fitting of differential equation systems. Rate constants determined for simpler reactions were fixed in the more complex systems. A 13-step model with the three above-mentioned rapid equilibria fits the - -3 - -3 - - overall reaction and all of its subsystems over the range [I ]0 < 10 M, [ClO2 ]0 < 10 M, [I ]0/[ClO2 ]0 ) 3-5, pH ) 1-3.5, and 25 °C. -

A Fundamental Evaluation of the Atmospheric Pre-Leaching Section of the Nickel-Copper Matte Treatment Process
A FUNDAMENTAL EVALUATION OF THE ATMOSPHERIC PRE-LEACHING SECTION OF THE NICKEL-COPPER MATTE TREATMENT PROCESS by RODRICK MULENGA LAMYA Dissertation presented for the Degree of DOCTOR OF PHILOSOPHY (Extractive Metallurgical Engineering) in the Department of Process Engineering at the University of Stellenbosch, South Africa Promoter Prof. L. Lorenzen STELLENBOSCH March 2007 DECLARATION I the undersigned, hereby declare that the work contained in this dissertation is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. Signature: ............................................... Date: ....................................................... Copyright © 2007 Stellenbosch University All rights reserved i SYNOPSIS Nickel-Copper sulphide ores are the most important Platinum Group Metal bearing ores. The South African deposits are exceptionally rich in the platinum group metals (PGMs) and production of the PGMs is the primary purpose of treating these ores. The methods used in the recovery of the PGMs from the nickel-copper ores generally consists of ore concentration by physical techniques, pyrometallurgical concentration and hydrometallurgical extraction of the base metals followed by the PGMs. Pyrometallurgical concentration produces Ni-Cu matte, which is treated by hydrometallurgical processes to recover the nickel, copper, cobalt and the precious metals. In this study, the leaching behaviour of a Ni–Cu matte in CuSO4–H2SO4 solution during the repulping (pre-leach) stage at Impala Platinum Refineries was studied. The repulping stage is basically a non–oxidative atmospheric leach stage, in which nickel, iron and cobalt are partially dissolved, while the copper is precipitated. To understand the nature of the leaching process during this stage of the base metal refining operation, the effects of variations in the key process variables such as temperature, stirring rate, particle size, pulp density, residence time, initial copper and acid concentrations were investigated. -

Cyanide Remediation: Current and Past Technologies C.A
CYANIDE REMEDIATION: CURRENT AND PAST TECHNOLOGIES C.A. Young§ and T.S. Jordan, Department of Metallurgical Engineering, Montana Tech, Butte, MT 59701 ABSTRACT Cyanide (CN-) is a toxic species that is found predominantly in industrial effluents generated by metallurgical operations. Cyanide's strong affinity for metals makes it favorable as an agent for metal finishing and treatment and as a lixivant for metal leaching, particularly gold. These technologies are environmentally sound but require safeguards to prevent accidental spills from contaminating soils as well as surface and ground waters. Various methods of cyanide remediation by separation and oxidation are therefore reviewed. Reaction mechanisms are given throughout. The methods are compared in regard to their effectiveness in treating various cyanide species: free cyanide, thiocyanate, weak-acid dissociables and strong-acid dissociables. KEY WORDS cyanide, metal-cyanide complex, thiocyanate, oxidation, separation INTRODUCTION ent on the transport of these heavy metals through their tissues, cyanide is very toxic. Waste waters from industrial operations The mean lethal dose to the human adult is transport many chemicals that have ad- between 50 and 200 mg [2]. U.S. EPA verse effects on the environment. Various standards for drinking and aquatic-biota chemicals leach heavy metals which would waters regarding total cyanide are 200 and otherwise remain immobile. The chemicals 50 ppb, respectively, where total cyanide and heavy metals may be toxic and thus refers to free and metal-complexed cya- cause aquatic and land biota to sicken or nides [3]. According to RCRA, all cyanide species are considered to be acute haz- die. Most waste-water processing tech- ardous materials and have therefore been nologies that are currently available or are designated as P-Class hazardous wastes being developed emphasize the removal of when being disposed of. -
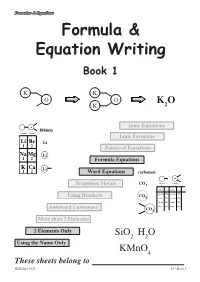
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions.