Acids and Bases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 8,569,369 B2 Kramer Et Al
USOO856.9369B2 (12) United States Patent (10) Patent No.: US 8,569,369 B2 Kramer et al. (45) Date of Patent: *Oct. 29, 2013 (54) AMINO ACID COMPOUNDS OTHER PUBLICATIONS (75) Inventors: Ronald Kramer, Phoenix, AZ (US); Jablecka et al., MedSci Monit 10(I):CR29-32 (2004). Alexander Nikolaidis, New Kallikratia Maynard et al., J. Nutr. 131:287-290 (2001). (GR) Ruel et al., J. Thorac Cardiovasc. Surg 135:762-77, 2008. Rytlewski et al., European Journal of Obstetrics & Gynecology and (73) Assignee: Thermolife International, LLC, Reproductive Biology 138:23-28 (2008). Phoenix, AZ (US) Schwedheim et al., Br J Clin Pharmacol 65(1):51-59 (2007). Smith et al., J. Thorac Cardiovasc. Surg 132:58-65 (2006). (*) Notice: Subject to any disclaimer, the term of this Rytlewski et al., Eur J. Clin Invest 35 (1):32-37 (2005). patent is extended or adjusted under 35 Ming et al., Circulation 110:3708-3714 (2004). U.S.C. 154(b) by 0 days. Romero et al., Cardiovascular Drug Reviews 24(3-4):275-290 (2006). This patent is Subject to a terminal dis Oka et al., Vasc Med 10:265-274 (2005). claimer. Hayashi et al., PNAS 102(38): 13681-13686 (2005). Grasemann et al., Eur Respir J 25:62-68 (2005). (21) Appl. No.: 13/468,231 Boger, J. Nutr 137:1650S-1655S (2007). Beghetti et al., J. Thorac Cardiovasc. Surg 132(6): 1501-1502 (2006). (22) Filed: May 10, 2012 Larsen et al., B. Acta Physiol 191(1):59-66 (2007). Berge, Journal of Pharmaceutical Sciences, Jan. 1977, vol. -
Aqueous Equilibrium Constants
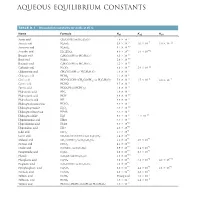
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

Guidance for Identification and Naming of Substance Under REACH
Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 GUIDANCE Guidance for identification and naming of substances under REACH and CLP May 2017 Version 2.1 2 Guidance for identification and naming of substances under REACH and CLP Version 2.1 - May 2017 LEGAL NOTICE This document aims to assist users in complying with their obligations under the REACH and CLP regulations. However, users are reminded that the text of the REACH and CLP Regulations is the only authentic legal reference and that the information in this document does not constitute legal advice. Usage of the information remains under the sole responsibility of the user. The European Chemicals Agency does not accept any liability with regard to the use that may be made of the information contained in this document. Guidance for identification and naming of substances under REACH and CLP Reference: ECHA-16-B-37.1-EN Cat. Number: ED-07-18-147-EN-N ISBN: 978-92-9495-711-5 DOI: 10.2823/538683 Publ.date: May 2017 Language: EN © European Chemicals Agency, 2017 If you have any comments in relation to this document please send them (indicating the document reference, issue date, chapter and/or page of the document to which your comment refers) using the Guidance feedback form. The feedback form can be accessed via the EVHA Guidance website or directly via the following link: https://comments.echa.europa.eu/comments_cms/FeedbackGuidance.aspx European Chemicals Agency Mailing address: P.O. Box 400, FI-00121 Helsinki, Finland Visiting address: Annankatu 18, Helsinki, Finland Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 PREFACE This document describes how to name and identify a substance under REACH and CLP. -

Cyanide Remediation: Current and Past Technologies C.A
CYANIDE REMEDIATION: CURRENT AND PAST TECHNOLOGIES C.A. Young§ and T.S. Jordan, Department of Metallurgical Engineering, Montana Tech, Butte, MT 59701 ABSTRACT Cyanide (CN-) is a toxic species that is found predominantly in industrial effluents generated by metallurgical operations. Cyanide's strong affinity for metals makes it favorable as an agent for metal finishing and treatment and as a lixivant for metal leaching, particularly gold. These technologies are environmentally sound but require safeguards to prevent accidental spills from contaminating soils as well as surface and ground waters. Various methods of cyanide remediation by separation and oxidation are therefore reviewed. Reaction mechanisms are given throughout. The methods are compared in regard to their effectiveness in treating various cyanide species: free cyanide, thiocyanate, weak-acid dissociables and strong-acid dissociables. KEY WORDS cyanide, metal-cyanide complex, thiocyanate, oxidation, separation INTRODUCTION ent on the transport of these heavy metals through their tissues, cyanide is very toxic. Waste waters from industrial operations The mean lethal dose to the human adult is transport many chemicals that have ad- between 50 and 200 mg [2]. U.S. EPA verse effects on the environment. Various standards for drinking and aquatic-biota chemicals leach heavy metals which would waters regarding total cyanide are 200 and otherwise remain immobile. The chemicals 50 ppb, respectively, where total cyanide and heavy metals may be toxic and thus refers to free and metal-complexed cya- cause aquatic and land biota to sicken or nides [3]. According to RCRA, all cyanide species are considered to be acute haz- die. Most waste-water processing tech- ardous materials and have therefore been nologies that are currently available or are designated as P-Class hazardous wastes being developed emphasize the removal of when being disposed of. -

WL-13 Safety Data Sheet, Canada (SDS-WL-13-CA
WL- 13 Safety Data Sheet according to the Hazardous Products Regulation (February 11, 2015) SECTION 1: Identification 1.1. Product identifier Product form : Mixture Product name : WL- 13 1.2. Recommended use and restrictions on use No additional information available 1.3. Supplier Swagelok Supplier: 29495 F.A. Lennon Drive Distributor, add your contact information 44139 Solon, OH - United States T 440-349-5600 - F 440-519-3304 www.swagelok.com 1.4. Emergency telephone number Emergency number : Infotrac: North America: 1-800-535-5053 International: 1-352-323-3500 SECTION 2: Hazard identification 2.1. Classification of the substance or mixture Classification (GHS-CA) Not classified 2.2. GHS Label elements, including precautionary statements GHS-CA labeling No labeling applicable 2.3. Other hazards No additional information available 2.4. Unknown acute toxicity (GHS-CA) No data available SECTION 3: Composition/Information on ingredients 3.1. Substances Not applicable 3.2. Mixtures Name Chemical name / Synonyms Product identifier % Classification (GHS-CA) Molybdenum(IV) sulfide Molybdenum disulfide / Molybdenum (CAS-No.) 1317-33-5 <= 10 Comb. Dust disulphide / Molybdenum sulfide / Molybdenum sulfide (MoS2) / Manganese tetroxide Sodium nitrite Diazotizing salts / Nitrous acid, (CAS-No.) 7632-00-0 <= 1.8 Acute Tox. 3 (Oral), H301 sodium salt / Nitrous acid, sodium Aquatic Acute 1, H400 salt (1:1) / SODIUM NITRITE Full text of hazard classes and H-statements: see section 16 SECTION 4: First-aid measures 4.1. Description of first aid measures First-aid measures after inhalation : Allow victim to breathe fresh air. Allow the victim to rest. First-aid measures after skin contact : Remove affected clothing and wash all exposed skin area with mild soap and water, followed by warm water rinse. -

Mechanisms of Nitric Oxide Reactions Mediated by Biologically Relevant Metal Centers
Struct Bond (2014) 154: 99–136 DOI: 10.1007/430_2013_117 # Springer-Verlag Berlin Heidelberg 2013 Published online: 5 October 2013 Mechanisms of Nitric Oxide Reactions Mediated by Biologically Relevant Metal Centers Peter C. Ford, Jose Clayston Melo Pereira, and Katrina M. Miranda Abstract Here, we present an overview of mechanisms relevant to the formation and several key reactions of nitric oxide (nitrogen monoxide) complexes with biologically relevant metal centers. The focus will be largely on iron and copper complexes. We will discuss the applications of both thermal and photochemical methodologies for investigating such reactions quantitatively. Keywords Copper Á Heme models Á Hemes Á Iron Á Metalloproteins Á Nitric oxide Contents 1 Introduction .................................................................................. 101 2 Metal-Nitrosyl Bonding ..................................................................... 101 3 How Does the Coordinated Nitrosyl Affect the Metal Center? .. .. .. .. .. .. .. .. .. .. .. 104 4 The Formation and Decay of Metal Nitrosyls ............................................. 107 4.1 Some General Considerations ........................................................ 107 4.2 Rates of NO Reactions with Hemes and Heme Models ............................. 110 4.3 Mechanistic Studies of NO “On” and “Off” Reactions with Hemes and Heme Models ................................................................................. 115 4.4 Non-Heme Iron Complexes .......................................................... -

Amino-Nitrogen Contents of Wool and Collagen
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP787 Part of Journal of Research of the National Bureau of Standards, Volume 14, May 1935 AMINO-NITROGEN CONTENTS OF WOOL AND COLLAGEN By Joseph R. Kanagy 1 and Milton Harris 2 ABSTRACT When wool, collagen, and arginine were treated with nitrous acid, increasing amounts of nitrogen were evolved with time. The continued evolution of nitro gen was due to the action of nitrous acid on the guanidine nuclei of these materials. A new method for the determination of the arginine content of a protein is given. The method is based on the relative rates of evolution of nitrogen from the guanidine nuclei in a protein and in arginine. Evidence is presented t o show that the action of nitrous acid on the guanidine nucleus is different from its action on a free amino group. The free amino-nitro gen contents of wool and collagen were calculated by subtracting from the total nitrogen evolved that portion of nitrogen which came from the guanidine nuclei. The values obtained for the percentages of the total nitrogen as amino nitrogen are 2.53 for wool and 2.77 for collagen. CONTENTS Page I. Introduction ___ _____________ __________________________________ _ 563 II. Materials and methods _____________________________________ ____ _ 564 III. 566 IV. 569 Conclu~on _____________________________ __ _____________________ _ V. ~frc~~~~~~~~~~======================= ===== ========= =========== 573 VI. References __ _____ ___________ _____ ___________________ _______ ___ _ 573 I. INTRODUCTION The free amino groups in wool and collagen have been related to the combination of these materials with acids, dyes, and tannins by various investigators. -

Combining Sodium Bicarbonate and Lidocaine Injection
Combining Sodium Bicarbonate and Lidocaine injection Tom Simpleman Consultant Pharmacist the FAWKS company http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f9c826a7‐8f19‐4857‐b5be‐11dcafcfc7a9 Referenced information from National Institutes of Health DESCRIPTION: Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution is a sterile, nonpyrogenic, solution of sodium bicarbonate (NaHCO3) in Water for Injection. It is added to an appropriate local anesthetic as a neutralizing agent immediately prior to administration. The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for single-use. pH is adjusted with carbon dioxide. Per the USP monograph for Sodium Bicarbonate Inj., pH is between 7.0 and 8.5. Osmolar concentration is 2 mOsmol/mL (calc.). Sodium bicarbonate, 84 mg is equal to one milliequivalent each of Na+ and HCO3-. Sodium Bicarbonate, USP is chemically designated as NaHC03, a white crystalline powder soluble in water. Sodium bicarbonate in water dissociates to provide sodium (Na+) and bicarbonate (HCO3-) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Bicarbonate (HCO3-) is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mEq/liter. Bicarbonate anion is considered “labile” since at a proper concentration of hydrogen ion (H+) it may be converted to carbonic acid (H2CO3) and thence to its volatile form, carbon dioxide (CO2) excreted by the lung. Normally a ratio of 1:20 (carbonic acid; bicarbonate) is present in the extracellular fluid. In a healthy adult with normal kidney function, practically all the glomerular filtered bicarbonate ion is reabsorbed; less than 1% is excreted in the urine. -

Important Prescribing Information
Important Prescribing Information Subject: Temporary importation of 8.4% Sodium Bicarbonate Injection to address drug shortage issues June 14, 2019 Dear Healthcare Professional, Due to the current critical shortage of Sodium Bicarbonate Injection, USP in the United States (US) market, Athenex Pharmaceutical Division, LLC (Athenex) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of Sodium Bicarbonate Injection. Athenex has initiated temporary importation of another manufacturer’s 8.4% Sodium Bicarbonate Injection (1 mEq/mL) into the U.S. market. This product is manufactured and marketed in Australia by Phebra Pty Ltd (Phebra). At this time, no other entity except Athenex Pharmaceutical Division, LLC is authorized by the FDA to import or distribute Phebra’s 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, in the United States. FDA has not approved Phebra’s 8.4% Sodium Bicarbonate Injection but does not object to its importation into the United States. Effective immediately, and during this temporary period, Athenex will offer the following presentation of Sodium Bicarbonate Injection: Sodium Bicarbonate Injection, 8.4% (1mEq/mL), 10mL per vial, 10 vials per carton Ingredients: sodium bicarbonate, water for injection, disodium edetate and sodium hydroxide (pH adjustment) Marketing Authorization Number in Australia is: 131067 Phebra’s Sodium Bicarbonate Injection contains the same active ingredient, Sodium Bicarbonate, in the same strength and concentration, 8.4% (1 mEq/mL) as the U.S. registered Sodium Bicarbonate Injection, USP by Pfizer’s subsidiary, Hospira. However, it is important to note that Phebra’s Sodium Bicarbonate Injection (1 mEq/mL), is provided only in a Single Use 10 mL vials, whereas Hospira’s product is provided in 50 mL single-dose vials and syringes. -

UNITED STATES PATENT OFFICE. ">Co
UNITED STATES PATENT OFFICE. LEONHARD LEDERER, OF MUNICH, GERMANY. PROCESS OF PRE PARNG HAO D DERVATIVES OF ACET ONE. SPECIFICATION forming part of Letters Patent No. 643,144, dated February 13, 1900. Application filed August 10, 1897, Serial No. 647,759. (No specimens.) To all livhon, it nay conce77: iodacetone. Also if not kept dry it some Beit known that I, LEONHARDLEDERER, a times undergoes decomposition at an ordi citizen of the Kingdom of Bavaria, residing nary temperature, thereby evolving so much SO at Munich, Bavaria, Germany, have invented heat that vapor of iodin is set free. Alco a new and useful Process of Preparing the hol, ether, and most of the organic solvents IIalogen Derivatives of Acetone, of which the set free iodin from per-iod-acetone. With following is a specification. dilute soda-lye it forms iodoform in the cold. 55 It is well known that the halogens act read The action of iodin on acetone-dicarbonic. ily upon acetonedicarbonic acid. For in acid can be so regulated by definite diminu stance, bromin added to an aqueous solution tions of the quantity of iodin added as to re of acetonedicarbonic acid is immediately sult in lower stages of iodin combination with taken up. An aqueous solution of acetonedi acetone. With regard to its behavior toward carbonic acid decomposed with an alcoholic alcohol and ether the penta-iod derivative is iodin solution takes up quickly the color of in close relation with the periodacetone. On the latter without visible reaction. If, how the addition of dilute soda-lye it is trans ever, this mixture be gently Warmed, reac formed into iodoform even in the cold, but tion takes place, evolving carbonic acid gas; not with the soda solution. -

Process for the Preparation of Chacogens and Chalcogenide Alloys of Controlled Average Crystallite Size
Europaisches Patentamt European Patent Office @ Publication number: 0 1 60 493 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 85302825.6 © Int. CI.4: C 01 B 19/02 _ C 01 B 19/04 © Date of filing: 23.04.85 © Priority: 23.04.84 US 603019 © Applicant: XEROX CORPORATION Xerox Square - 020 Rochester New York 14644(US) @ Date of publication of application: 06.11.85 Bulletin 85/45 @ Inventor: Badesha, Santokh Singh 665 Cievenger Road @ Designated Contracting States: Ontario New York 1451 9(US) DE FR GB © Inventor: Fekete, George Thomas 111 Dale Road Rochester New York 14625IUS) © Representative: Goode, Ian Roy et al, European Patent Attorney do Rank Xerox Limited Patent Department Rank Xerox House 338 Euston Road London NW1 3BH(GB) (64) Process for the preparation of chacogens and chalcogenide alloys of controlled average crystallite size. A process for the preparation of chalcogens and chal- cogenide alloys which comprises subjecting the correspond- ing esters to a reduction or coreduction reaction, for example using hydrazine, at a temperature between about 40°C and 135°C. The morphology/crystallite size of the product is controlled by suitably selecting a temperature within this range, depending on the particular chalcogen or alloy being prepared. The product is either a non-crystalline material, or has an average crystallite size between about 15 and 52 nm. This invention relates to a process for the preparation of chalcogens and chalcogenide alloys, the process being of the kind which comprises subjecting the corresponding esters to a reduction or coreduction reaction. Such a process for preparing high purity tellurium is described in EP-A-0 102 753, and such a process for preparing high purity chalcogenide alloys, such as selenium/tellurium alloys, is described in EP-A-0 101 238. -

Structure and Functioning of the Acid–Base System in the Baltic Sea
Earth Syst. Dynam., 8, 1107–1120, 2017 https://doi.org/10.5194/esd-8-1107-2017 © Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Structure and functioning of the acid–base system in the Baltic Sea Karol Kulinski´ 1, Bernd Schneider2, Beata Szymczycha1, and Marcin Stokowski1 1Institute of Oceanology, Polish Academy of Sciences, IO PAN, ul. Powstanców´ Warszawy 55, 81-712 Sopot, Poland 2Leibniz Institute for Baltic Sea Research Warnemünde, IOW, Seestrasse 15, 18119 Rostock, Germany Correspondence: Karol Kulinski´ ([email protected]) Received: 6 April 2017 – Discussion started: 12 April 2017 Revised: 21 October 2017 – Accepted: 6 November 2017 – Published: 11 December 2017 Abstract. The marine acid–base system is relatively well understood for oceanic waters. Its structure and func- tioning is less obvious for the coastal and shelf seas due to a number of regionally specific anomalies. In this review article we collect and integrate existing knowledge of the acid–base system in the Baltic Sea. Hydro- graphical and biogeochemical characteristics of the Baltic Sea, as manifested in horizontal and vertical salinity gradients, permanent stratification of the water column, eutrophication, high organic-matter concentrations and high anthropogenic pressure, make the acid–base system complex. In this study, we summarize the general knowledge of the marine acid–base system as well as describe the peculiarities identified and reported for the Baltic Sea specifically. In this context we discuss issues such as dissociation constants in brackish water, differ- ent chemical alkalinity models including contributions by organic acid–base systems, long-term changes in total alkalinity, anomalies of borate alkalinity, and the acid–base effects of biomass production and mineralization.