Process for the Preparation of Chacogens and Chalcogenide Alloys of Controlled Average Crystallite Size
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
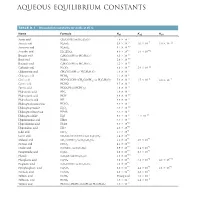
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

Acids and Bases
Name Date Class CHAPTER 14 REVIEW Acids and Bases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Name the following compounds as acids: sulfuric acid a. H2SO4 sulfurous acid b. H2SO3 hydrosulfuric acid c. H2S perchloric acid d. HClO4 hydrocyanic acid e. hydrogen cyanide 2. H2S Which (if any) of the acids mentioned in item 1 are binary acids? 3. Write formulas for the following acids: HNO2 a. nitrous acid HBr b. hydrobromic acid H3PO4 c. phosphoric acid CH3COOH d. acetic acid HClO e. hypochlorous acid 4. Calcium selenate has the formula CaSeO4. H2SeO4 a. What is the formula for selenic acid? H2SeO3 b. What is the formula for selenous acid? 5. Use an activity series to identify two metals that will not generate hydrogen gas when treated with an acid. Choose from Cu, Ag, Au, Pt, Pd, or Hg. 6. Write balanced chemical equations for the following reactions of acids and bases: a. aluminum metal with dilute nitric acid ϩ → ϩ 2Al(s) 6HNO3(aq) 2Al(NO3)3(aq) 3H2(g) b. calcium hydroxide solution with acetic acid ϩ → ϩ Ca(OH)2(aq) 2CH3COOH(aq) Ca(CH3COO)2(aq) 2H2O(l ) MODERN CHEMISTRY ACIDS AND BASES 117 Copyright © by Holt, Rinehart and Winston. All rights reserved. Name Date Class SECTION 1 continued 7. Write net ionic equations that represent the following reactions: a. the ionization of HClO3 in water ϩ → ϩ ϩ Ϫ HClO3(aq) H2O(l ) H3O (aq) ClO3 (aq) b. NH3 functioning as an Arrhenius base ϩ → ϩ ϩ Ϫ NH3(aq) H2O(l ) ← NH4 (aq) OH (aq) 8. -

1,2-Cyclohexanedione Dioxime
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

H2seo3. Arxiv:1901.03765V1
Crystal Structure Elucidation of the Novel Molecular-Inorganic Polymer K2SeO4 · H2SeO3. Oscar S. Hernández-Daguer 1,2, Charles L. Barnes3, Samuel P. Hernández-Rivera4, and Jorge L. Ríos-Steiner 5 1Department of Physics, University of Massachusetts, Amherst, Massachusetts 01003, United States, e-mail: [email protected], [email protected] 2Department of Physics, University of Puerto Rico, Mayagüez, Puerto Rico 00681-9000, United States, e-mail: [email protected], [email protected], phone: +1 787 228 1380. 3Department of Chemistry, University of Missouri, Columbia, Missouri 65211, United States. 4ALERT-II DHS Center of Excellence for Explosives Research, Department of Chemistry, University of Puerto Rico, Mayagüez, Puerto Rico, 00681-9000, United States. 5Laboratory of Crystallography and Synthesis of New Materials, Department of Chemistry, University of Puerto Rico, Mayagüez, Puerto Rico, 00681-9000 United States, e-mail: [email protected], phone: +1 787 832 4040 ext 2538, Fax: +1 787 265 3849. January 15, 2019 Abstract The orthorhombic crystal structure of the novel molecular-inorganic polymer K2SeO4 · H2SeO3 was elucidated using single-crystal X-ray diffraction with MoKα radiation (λ= 0.71073 Å), performed at 100 and 298.15 K. The reported data is at 100 K since there were no struc- tural differences as compared to the room temperature. The technique revealed K2SeO4 ·H2SeO3 crystals to have space group P bcm with unit cell dimensions a = 8.8672(17) Å, b = 7.3355(14) Å, c = 11.999(2) Å and Z = 4. The unit cell volume obtained was V = 780.5(3) Å3 3 arXiv:1901.03765v1 [cond-mat.mtrl-sci] 11 Jan 2019 with a calculated density Dc = 2:980Mg=m . -

WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61P 3/04 (2006.01) A61K 33/40 (2006.01) kind of national protection available): AE, AG, AL, AM, A61P 9/10 (2006.01) A61K 38/44 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 35/74 (2015.01) A61K 31/17 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/US20 16/034973 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 3 1 May 2016 (3 1.05.2016) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/169,480 1 June 2015 (01 .06.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/327,283 25 April 2016 (25.04.2016) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: XENO BIOSCIENCES INC. -

Preparation of Monodisperse Se Colloid Spheres and Se Nanowires
Nano Res (2008) 1: 403 411 DOI 10.1007/s12274-008-8040-5 00403 Research Article Preparation of Monodisperse Se Colloid Spheres and Se Nanowires Using Na2SeSO3 as Precursor Liping Liu, Qing Peng, and Yadong Li( ) Department of Chemistry, Tsinghua University, Beijing 100084, China Received: 3 September 2008 / Revised: 19 September 2008 /Accepted: 19 September 2008 ©Tsinghua Press and Springer-Verlag 2008. This article is published with open access at Springerlink.com ABSTRACT Nearly monodisperse spherical amorphous Se colloids are prepared by the dismutation of Na2SeSO3 solution at room temperature; by altering the pH of the solution, amorphous Se colloid spheres with sizes of about 120 nm, 200 nm, 300 nm, and 1 μm can be obtained. Se@Ag2Se core/shell spheres are successfully synthesized by using the obtained amorphous Se (a-Se) spheres as templates, indicating the potential applications of these Se nanomaterials in serving as soft templates for other selenides. Meanwhile, selenium nanowires are obtained through a “solid-solution-solid” growth process by dispersing the prepared Se spheres in ethanol. This simple and environmentally benign approach may offer more opportunities in the synthesis and applications of nanocrystal materials. KEYWORDS Na2SeSO3, dismutation, amorphous Se (a-Se) spheres, trigonal Se (t-Se) nanowires Introduction structures, as it is easily removed [4, 5]. Furthermore, selenium is a relatively active material and can react Materials which are composed of the element selenium with a variety of chemical reagents to form other play important roles in the fields of photonics materials, such as Ag Se, and CdSe [1, 6, 7], which and electronics due to its special photoconductive 2 possess wide potential applications as soft templates properties [1, 2]; the electrical conductivity of selenium for functional selenides. -

(Ii) 3,870,614 Underwood (45) Mar
United States Patent 19 (ii) 3,870,614 Underwood (45) Mar. 11, 1975 54 SELENIUM DEPOSITION Primary Examiner-Howard S. Williams 75 Inventor: John Duckles Underwood, Bishop Attorney, Agent, or Firm-John T. O'Halloran; Stortford, England Menotti J. Lombardi, Jr.; Vincent Ingrassia 73) Assignee: International Standard Electric Corporation, New York, N.Y. (22 Filed: June 6, 1974 57 ABSTRACT 21 Appl. No.: 476,986 This relates to a method of depositing selenium in a suitable form for the manufacture of rectifiers. Sele (52) U.S. Cl. ................................................ 204181 nium is electrophoretically deposited from a mixture (51) int. Cl............................................... B01k 5/02 of two selenium sols. The first sol is prepared by 58) Field of Search..................................... 204/18 chemical reduction of selenous acid and the second by grinding and colloid milling metallic selenium. 56 References Cited UNITED STATES PATENTS 8 Claims, 1 Drawing Figure 3,745,098 7, 1973 Brown et al........................ 2041 181 GREY SELENUM HEAT TREATMENT RECYCLE GRND AND COLLOD MLL WASH AND DECANT DSPERSE IN SO MEDUM ELECTROPHORESS TENTED KAR 1975 3,87O64. GREY SELENUM HEAT TREATMENT RECYCLE GRND AND COLLOD MLL WASH AND DECANT OSPERSE IN SOL MEDUM ELECTROPHORESS 3,870,614 1. 2 SELENIUM DEPOSITION After milling several times the colloidal particles are allowed to settle, the liquid is decanted and the parti BACKGROUND OF THE INVENTION cles are washed by decantation with an alcohol such as This invention relates to the electrophoretic deposi ethanol. The colloidal particles are then formed into a tion of selenium and in particular to a method of depos sol by dispersion in a sol medium typically consisting iting selenium in a form suitable for manufacturing rec of: tifiers. -

Safety Data Sheet
Safety Data Sheet Better Chemistry. Better Business BLACK-MAGIC® RT SS4 Revised: 6/28/21 1 IDENTIFICATION Product Name: BLACK-MAGIC® RT SS4 Product Code :2260002 Recommended use of the chemical and restrictions on use:Industrial applications Hubbard-Hall Inc. 563 South Leonard Street Waterbury, CT 06708 Telephone: 203-756-5521 Fax number: 203-756-9017 Emergency Phone Number CHEMTREC: 1 (800) 424-9300 International: 1 (703) 527-3887 2 HAZARDS IDENTIFICATION Signal Word: DANGER Hazard Category: Acute Toxicity-Oral Hazard Category 4 Eye Damage/Irritation Hazard Category 1 Skin Corrosion/Irritation Hazard Category 1A Acute Toxicity-Inhalation Hazard Category 4 Specific Target Organ Toxicity (Single Exposure) Hazard Category 3 Corrosive to Metals Hazard Category 1 Acute Aquatic Toxicity-Category 2 Hazard Statements: Harmful if swallowed or inhaled. Causes severe skin burns and eye damage. May be corrosive to metals. May cause respiratory irritation. Toxic to aquatic life Prevention: Wash skin thoroughly after handling. Do not eat, drink or smoke when using this product. Avoid breathing dust, fumes, gas, mist, vapors and sprays. Wear rubber gloves, goggles and chemical protective clothing. Use only outdoors or in well ventilated area. Keep only in original container. Response: If swallowed: Rinse mouth. Do NOT induce vomiting. Page 1 of 5 2260002 BLACK-MAGIC® RT SS4 If on skin (or hair): Take off immediately all contaminated clothing Rinse skin with water/shower . Wash contaminated clothing before reuse. If inhaled: Remove person to fresh air and keep comfortable for breathing. Call poison center/doctor if you feel unwell. Immediately call poison center or doctor and explain the type of exposure to the chemical(s) and provide the name of the chemical(s). -

Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review
molecules Review Riley Oxidation of Heterocyclic Intermediates on Paths to Hydroporphyrins—A Review Pengzhi Wang and Jonathan S. Lindsey * Department of Chemistry, North Carolina State University, Raleigh, NC 27695-8204, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-515-6406 Academic Editors: M. Graça P. M. S. Neves, M. Amparo F. Faustino and Nuno M. M. Moura check for updates Received: 22 March 2020; Accepted: 16 April 2020; Published: 17 April 2020 Abstract: Riley oxidation of advanced heterocyclic intermediates (dihydrodipyrrins and tetrahydrodipyrrins) is pivotal in routes to synthetic hydroporphyrins including chlorins, bacteriochlorins, and model (bacterio)chlorophylls. Such macrocycles find wide use in studies ranging from energy sciences to photomedicine. The key transformation (–CH3 → –CHO) is often inefficient, however, thereby crimping the synthesis of hydroporphyrins. The first part of the review summarizes 12 representative conditions for Riley oxidation across diverse (non-hydrodipyrrin) substrates. An interlude summarizes the proposed mechanisms and provides context concerning the nature of various selenium species other than SeO2. The second part of the review comprehensively reports the conditions and results upon Riley oxidation of 45 1-methyltetrahydrodipyrrins and 1- methyldihydrodipyrrins. A comparison of the results provides insights into the tolerable structural features for Riley oxidation of hydrodipyrrins. In general, Riley oxidation of dihydrodipyrrins has a broad scope toward substituents, but proceeds in only modest yield. Too few tetrahydrodipyrrins have been examined to draw conclusions concerning scope. New reaction conditions or approaches will be required to achieve high yields for this critical transformation in the synthesis of hydroporphyrins. Keywords: aldehyde; bacteriochlorin; chlorin; dihydrodipyrrin; dipyrromethane; selenium dioxide; selenium reagent; tetrahydrodipyrrin 1. -

Common Name: SELENIOUS ACID HAZARD SUMMARY
Common Name: SELENIOUS ACID CAS Number: 7783-00-8 RTK Substance number: 2762 DOT Number: UN 3283 Date: September 1999 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Selenious Acid can affect you when breathed in and by * Exposure to hazardous substances should be routinely passing through your skin. evaluated. This may include collecting personal and area * Contact can irritate and burn the skin and eyes. air samples. You can obtain copies of sampling results * Breathing Selenious Acid can irritate the nose and throat. from your employer. You have a legal right to this * Breathing Selenious Acid can irritate the lungs causing information under OSHA 1910.1020. coughing and/or shortness of breath. Higher exposures * If you think you are experiencing any work-related health can cause a build-up of fluid in the lungs (pulmonary problems, see a doctor trained to recognize occupational edema), a medical emergency, with severe shortness of diseases. Take this Fact Sheet with you. breath. * Exposure can cause headache, nausea, vomiting, WORKPLACE EXPOSURE LIMITS abdominal pain, pallor and fatigue. The following exposure limits are for Selenious Acid * Repeated exposure can cause garlic odor of the breath, (measured as Selenium): metallic taste, increased dental cavities, and loss of hair and nails. OSHA: The legal airborne permissible exposure limit * Repeated exposure may cause personality changes of (PEL) is 0.2 mg/m3 averaged over an 8-hour depression, anxiety or irritability. workshift. * Selenious Acid can damage the nervous system. * Selenious Acid may damage the liver and kidneys. NIOSH: The recommended airborne exposure limit is 0.2 mg/m3 averaged over a 10-hour workshift. -

Selenium Dioxide Catalysed Oxidation of Acetic Acid Hydrazide by Bromate in Aqueous Hydrochloric Acid Medium
J. Chem. Sci. Vol. 124, No. 4, July 2012, pp. 821–826. c Indian Academy of Sciences. Selenium dioxide catalysed oxidation of acetic acid hydrazide by bromate in aqueous hydrochloric acid medium R S YALGUDRE and G S GOKAVI∗ Kinetics and Catalysis Laboratory, Department of Chemistry, Shivaji University Kolhapur 416 004, India e-mail: [email protected] MS received 8 June 2011; revised 31 August 2011; accepted 13 September 2011 Abstract. Selenium dioxide catalysed acetic acid hydrazide oxidation by bromate was studied in hydrochloric acid medium. The order in oxidant concentration, substrate and catalyst were found to be unity. Increasing hydrogen ion concentration increases the rate of the reaction due to protonation equilibria of the oxidant. The mechanism of the reaction involves prior complex formation between the catalyst and substrate, hydrazide, followed by its oxidation by diprotonated bromate in a slow step. Acetic acid was found to be the oxidation product. Other kinetic data like effect of solvent polarity and ionic strength on the reaction support the proposed mechanism. Keywords. Acetic acid hydrazide; oxidation; bromate; kinetics; selenium dioxide; catalysis. 1. Introduction offers an excellent opportunity to look into the mecha- nistic pathway. Hydrazides of carbonic acids are important starting The oxidative transformation of hydrazides, with materials in organic synthesis 1 as well as they find most of the oxidants used, give corresponding acids and applications in medicine and analytical chemistry. 2 in some cases 1 esters or amides. Hydrazides have also They also form coordination complexes with many been converted into N–N-diacylhydrazines by various transition metal ions which make them good reagents oxidants. -

Selenium Interim Final
Ecological Soil Screening Levels for Selenium Interim Final OSWER Directive 9285.7-72 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 July 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR SELENIUM ................................2 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................5 4.0 ECO-SSL FOR SOIL INVERTEBRATES....................................5 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................8 5.1 Avian TRV ........................................................8 5.2 Estimation of Dose and Calculation of the Eco-SSL .......................14 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE .................................14 6.1 Mammalian TRV ..................................................14 6.2 Estimation of Dose and Calculation of the Eco-SSL .......................23 7.0 REFERENCES .........................................................25 7.1 General Selenium References ........................................25 7.2 References for Plants and Soil Invertebrates .............................26 7.3 References Rejected for Use in Deriving Plant and Soil Invertebrate Eco-SSLs ...............................................................26 7.4 References Used in Deriving Wildlife TRVs ............................38 7.5 References Rejected for Use in Derivation of Wildlife TRV ................50 i LIST