4. Chemical and Physical Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

The Management of Common Skin Conditions in General Practice
Management of Common Skin Conditions In General Practice including the “red rash made easy” © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care University of Auckland, Tamaki Campus Reviewed by Hon A/Prof Amanda Oakley - 2019 http://www.dermnetnz.org Management of Common Skin Conditions In General Practice Contents Page Derm Map 3 Classic location: infants & children 4 Classic location: adults 5 Dermatology terminology 6 Common red rashes 7 Other common skin conditions 12 Common viral infections 14 Common bacterial infections 16 Common fungal infections 17 Arthropods 19 Eczema/dermatitis 20 Benign skin lesions 23 Skin cancers 26 Emergency dermatology 28 Clinical diagnosis of melanoma 31 Principles of diagnosis and treatment 32 Principles of treatment of eczema 33 Treatment sequence for psoriasis 34 Topical corticosteroids 35 Combination topical steroid + antimicrobial 36 Safety with topical corticosteroids 36 Emollients 37 Antipruritics 38 For further information, refer to: http://www.dermnetnz.org And http://www.derm-master.com 2 © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care, University of Auckland, Tamaki Campus. Management of Common Skin Conditions In General Practice DERM MAP Start Is the patient sick ? Yes Rash could be an infection or a drug eruption? No Insect Bites – Crop of grouped papules with a central blister or scab. Is the patient in pain or the rash Yes Infection: cellulitis / erysipelas, impetigo, boil is swelling, oozing or crusting? / folliculitis, herpes simplex / zoster. Urticaria – Smooth skin surface with weals that evolve in minutes to hours. No Is the rash in a classic location? Yes See our classic location chart . -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

A 1 Case-PR/ }*Rciofft.;Is Report
.A 1 case-PR/ }*rciofft.;is Report (a) This eruption site on Mauna Loa Volcano was the main source of the voluminous lavas that flowed two- thirds of the distance to the town of Hilo (20 km). In the interior of the lava fountains, the white-orange color indicates maximum temperatures of about 1120°C; deeper orange in both the fountains and flows reflects decreasing temperatures (<1100°C) at edges and the surface. (b) High winds swept the exposed ridges, and the filter cannister was changed in the shelter of a p^hoehoc (lava) ridge to protect the sample from gas contamination. (c) Because of the high temperatures and acid gases, special clothing and equipment was necessary to protect the eyes. nose, lungs, and skin. Safety features included military flight suits of nonflammable fabric, fuil-face respirators that are equipped with dual acidic gas filters (purple attachments), hard hats, heavy, thick-soled boots, and protective gloves. We used portable radios to keep in touch with the Hawaii Volcano Observatory, where the area's seismic activity was monitored continuously. (d) Spatter activity in the Pu'u O Vent during the January 1984 eruption of Kilauea Volcano. Magma visible in the circular conduit oscillated in a piston-like fashion; spatter was ejected to heights of 1 to 10 m. During this activity, we sampled gases continuously for 5 hours at the west edge. Cover photo: This aerial view of Kilauea Volcano was taken in April 1984 during overflights to collect gas samples from the plume. The bluish portion of the gas plume contained a far higher density of fine-grained scoria (ash). -

United States Patent (19) 11, 3,989,755 Mccoy Et Al
United States Patent (19) 11, 3,989,755 McCoy et al. (45) Nov. 2, 1976 54 PRODUCTION OF OXIMES BY THE (56) References Cited REACTION OF CARBON MONOXDE WITH UNITED STATES PATENTS NTROCOMPOUNDS 2,945,065 7/1960 Donaruma...................... 260/566 A 75) Inventors: John J. McCoy, Media; John G. 3,480,672 11/1969 Kober et al..................... 260/566 A Zajacek, Strafford, both of Pa.; Karl 3,734,964 5/1973 Knifton........................... 260/566. A E. Fuger, Therwil, Switzerland (73) Assignee: Atlantic Richfield Company, Los Primary Examiner-Gerald A. Schwartz Angeles, Calif. Attorney, Agent, or Firm-Delbert E. McCaslin 22 Filed: Feb. 20, 1975 (21) Appl. No.: 551,487 57) ABSTRACT Related U.S. Application Data Production of oximes (and ketones) by contacting at 63) Continuation-in-part of Ser. No. 372,457, June 21, elevated temperatures and pressures, a primary or sec 1973, abandoned. ondary saturated aliphatic nitrocompound with carbon monoxide in the presence of a catalyst comprising me 52 U.S. C. ........................ 260/566 A; 260/586 C; tallic selenium or inorganic selenium compounds and 260/586 R; 260/593 R a base. (51 int. Cl”........................................ C07C 131/04 58) Field of Search........ 260/566 A, 586 A, 586 R, 20 Claims, No Drawings 260/593 R 3,989,755 2 cycloaliphatic nitrocompound is contacted with carbon PRODUCTION OF OXIMES BY THE REACTION OF monoxide at temperatures in the range of from 50° to 200 C. under pressures in the range of from 10 atmo CARBON MONOXIDE WITH NITROCOMPOUNDS spheres to 200 atmospheres in the presence of a sele nium catalyst and a base. -

Differential Effects of Tranylcypromine and Imidazole on Mammary Carcinogenesis in Rats Fed Low and High Fat Diets1
[CANCER RESEARCH 49, 3168-3172, June 15, 1989] Differential Effects of Tranylcypromine and Imidazole on Mammary Carcinogenesis in Rats Fed Low and High Fat Diets1 David L. McCormick,2 Ann M. Spicer, and Jacqueline L. Hollister Life Sciences Department, IIT Research Institute, Chicago, Illinois 60616 ABSTRACT studies with this class of compounds used inhibitors of the cyclooxygenase pathway of arachidonic acid catabolism; exper Neoplastic development in the rat mammary gland can be suppressed iments performed in our laboratory and by Ip and coworkers by inhibition of the activity of several enzymes involved in eicosanoid biosynthesis. In order to investigate the potential utility of prostacyclin demonstrated that the postcarcinogen phase of rat mammary and thromboxane synthetases as targets for mammary cancer chemopre- carcinogenesis can be suppressed by dietary administration of vention, experiments were conducted to determine the influence of tran- indomethacin (7, 8) or flurbiprofen (9). However, although the ylcypromine (TCP), an inhibitor of prostacyclin synthetase, and ¡mida/ole anticarcinogenic activity of indomethacin is similar to that of (IMI), an inhibitor of thromboxane synthetase, on mammary carcinogen- more widely studied inhibitors of mammary carcinogenesis such esis induced in rats by 7V-methyl-/V-nitrosourea. Fifty-day-old female as retinyl acetate (10) and selenium (11), the dose levels of Sprague-Dawley |Hsd:SD(BR)l rats received a single s.c. dose of 0 or 40 indomethacin required for chemopreventive efficacy in rats are mg of .V-mcth>l-.Y-nitrosoiirea per kg of body weight. Beginning 7 days close to the threshold of lethal toxicity (12). For this reason, after carcinogen administration, groups of rats were fed isoenergetic, studies are ongoing to identify additional modifiers of arachi casein-based diets containing 3 or 20% corn oil (w/w), supplemented with donic acid metabolism whose administration provides an effec (per kg of diet) 10 mg of TCP, 1000 mg of IMI, or sucrose carrier only. -
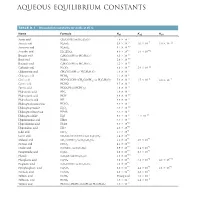
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in The
Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in the Presence of Selenite or Tellurite __________________ A Thesis Presented to The Faculty of the Department of Chemistry Sam Houston State University ____________________ In Partial Fulfillment Of the Requirements for the Degree of Master of Science ____________________ by Janet Horton Bius December, 2001 Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in the Presence of Selenite or Tellurite by Janet Horton Bius ____________________ Approved: _____________________________ Thomas G. Chasteen ____________________________ Mary F. Plishker ____________________________ Rick C. White Approved: ____________________________ Brian Chapman, Dean College of Arts and Sciences II ABSTRACT Bius, Janet Horton, Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in the Presence of Selenite or Tellurite, Master of Science (Chemistry), December, 2001. Sam Houston State University, Hunts- ville, Texas, 68 pp. Purpose The purpose of this investigation was to determine: (1) the mass balance of selenium or tellurium that was bioreduced when a selenium-resistant facultative anaerobe was amended with either selenium or tellurium; and (2) methods to analyze for these metalloids in biological samples. Methods Analytical methods were developed for the determination of -

Synthesis of Metal Selenide Semiconductor Nanocrystals Using Selenium Dioxide As Precursor
SYNTHESIS OF METAL SELENIDE SEMICONDUCTOR NANOCRYSTALS USING SELENIUM DIOXIDE AS PRECURSOR By XIAN CHEN A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2007 1 © 2007 Xian Chen 2 To my parents 3 ACKNOWLEDGMENTS Above all, I would like to thank my parents for what they have done for me through these years. I would not have been able to get to where I am today without their love and support. I would like to thank my advisor, Dr. Charles Cao, for his advice on my research and life and for the valuable help during my difficult times. I also would like to thank Dr. Yongan Yang for his kindness and helpful discussion. I learned experiment techniques, knowledge, how to do research and so on from him. I also appreciate the help and friendship that the whole Cao group gave me. Finally, I would like to express my gratitude to Dr. Ben Smith for his guidance and help. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ...............................................................................................................4 LIST OF FIGURES .........................................................................................................................7 ABSTRACT.....................................................................................................................................9 CHAPTER 1 SEMICONDUCTOR NANOCRYSTALS ............................................................................11 1.1 Introduction..................................................................................................................11 -

Diffraction of Selenium with Sulfide Solution and of Sulfur With
LFIDE SOL UTI01 UTIONS 1- known that selenium dissolves in solutions of alkali and alkaline-earth sulfides to form com- \yith niixed anions [I]. The solubility of seleniunl in sodium sulfide solutions is utilized in tech- -, . or example, sodium sulfide is used for extraction of selenium from sludge by-products in the . acid and pulp and paper industries (21. Several variants have been devised for smelting of dusts .~d-nianufacturingplants with formation of polysulfide slags containing selenium, which are then cd to further hydrometallurgical treatment [8, 41. There are also other possibiIities for effective ion of the properties of solutions of sodium selenosulfides. This makes it all the more necessary %, v.2,!y the interaction of selenium with sodium sulfides and of sulfur with solutions of sodium selenides, .kc. Information on the subject in the literature is very limited. abl le 1 contains data on the solubility of selenium in solutions of sodium mono- and polysulfides, ts! n the niain reactions occurring in the dissolution process. selenium and sulfur were dissolved in the various solutions in closed flasks, agitated by magnetic ,'.~:,.I*s.In order to avoid oxidation of the sulfides and selenides, the samples were withdrawn with the s c ! rubber bulb in a mechanical sampling device through an inverted Schott funnel contained in the reac- ;l.isk. Control experiments were conducted in an atmosphere of technical nitrogen additionally treated pyrogallol to remove oxygen. Sodium sulfide of analytical grade, sulfur containing 99.9% S, and selenium containing 99.99% Se were :W i in the experiments. The potentials were measured with an R-300 potentiometer with the aid of a platinum electrode, 11 .t 0.001 Q. -

Calculations of \(N,2N\) Reaction Cross Sections for 74,76,78,80,82Se up to 20
128 EPJ Web of Conferences , 01001 (2016) DOI: 10.1051/epjconf/201612801001 TESNAT 2016 Halide Şahan1,a, Muhittin Şahan1, Eyyup Tel1 1Osmaniye Korkut Ata University, Faculty of Arts and Science, Department of Physics, Osmaniye, Turkey In the present work, the excitation functions of (n,2n) reactions for five isotopes of selenium (74,76,78,80,82Se) are calculated using ALICE/ASH, EMPIRE-3.2.2, PCROSS, and TALYS 1.6 computer codes based on statistical model up to 20 MeV. The theoretical calculations provide information of the (n,2n) excitation functions with the increasing target neutron number of selenium element. The calculated cross-sections were compared with experimental data from EXFOR and also with the cross- sections estimated with semi empirical formula developed by Tet et al. (2008) [18]. Results show a reasonably good agreement between the calculations and the experimental data from literature. ! " by Tel et al. [13]. These formulas have been given as follows; The theoretical calculation models are necessary to ⎪⎧7.15[]1− 2.45e−31.620(N −Z ) / A for even A⎪⎫ provide the estimation of the particle–induced reaction σ = (1) ln n,2n ⎨ ⎬ cross sections due to the experimental difficulty [1,2]. In ⎩⎪ 7.65[]1−1.59e−23.06(N −Z ) / A for odd A⎭⎪ past years the cross section of selenium isotopes (74,76,78,80,82,84Se) around 14-15 MeV have been measured For (n,2n) reaction cross sections of 74Se(n,2n)73Se, by many researches such as Hille and Münzer [3]; 76Se(n,2n)75Se, 78Se(n,2n)77Se, 80Se(n,2n)79Se and Minetti and Pasquarelli [4]; Casanova and Sanchez [5]; 82Se(n,2n)81Se at 14-15 MeV, the calculated cross 74,76,78,80,82 Hoang et al. -

AN43285 – Accurate Determination of Arsenic and Selenium in Environmental Samples Using Triple Quadrupole ICP-MS
Certified for Thermo Scientific™ iCAP™ TQe ICP-MS APPLICATION NOTE 43285 Accurate determination of arsenic and selenium in environmental samples using triple quadrupole ICP-MS Authors: Marcus Manecki1, Simon Lofthouse2, Philipp Boening3 and Shona McSheehy Ducos1; 1Thermo Fisher Scientific, Bremen, Germany; 2Thermo Fisher Scientific, Hemel Hempstead, UK; 3Institute of Chemistry and Biology of the Marine Environment (ICBM), Carl von Ossietzky University of Oldenburg, Oldenburg, Germany Keywords: Arsenic, interference removal, Selenium for example is an essential element that is REE, rock, selenium, soil, sediment necessary for normal thyroid function and due to its antioxidant properties, is associated with several health Goal benefits. Diseases associated with selenium deficiency To demonstrate the accurate determination of arsenic such as Keshan disease and symptoms of hypothyroidism, and selenium in sediments and rocks that contain elevated are most commonly found in areas where levels of levels of rare earth elements using triple quadrupole selenium in soil are particularly low. Supplementation as a ICP-MS. remedy is common practice and is not isolated to humans. Understanding where soil selenium deficiencies occur for Introduction example supports the correct supplementation of cattle Due to the impact arsenic and selenium can have in the grazing in those areas to prevent white muscle disease environment at low levels, as a toxin or essential nutrient (a cattle specific selenium deficiency disease). respectively, it is important to be able to quantify them accurately. Arsenic on the other hand, in its inorganic forms Instrumentation (the most common forms found in ground water and soils) An iCAP TQ ICP-MS was used to analyse all samples.