Periodic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office Patented Apr
3,378,337 United States Patent Office Patented Apr. 16, 1968 1. 2 and the hydrogen peroxide. This result is achieved without 3,378,337 contaminating the reaction mixture. The final solution PREPARATHON OF ODEC ACID AND DERVATIVES THEREOF contains substantially pure iodic acid, and can be evap Ricardo O. Bach, Gastonia, N.C., assignor to Lithium orated and dehydrated to obtain the iodic acid, or the Corporation of America, Inc., New York, N.Y., a cor anhydride thereof, or it can be used as a medium for the poration of Minnesota direct production of salts of iodic acid. No Drawing. Fied May 17, 1965, Ser. No. 456,561 In carrying out the method of the present invention, 12 Claims. (CI. 23-85) the iodic acid solution employed in forming the reaction mixture should contain sufficient iodic acid to provide a O hydrogen ion and iodate ion concentration in the reaction ABSTRACT OF THE DISCLOSURE mixture which will favor oxidation of iodine to its penta A method of preparing solutions of substantially pure valent state and impede decomposition of hydrogen iodic acid from which salts of the acid, the anhydride peroxide. Generally speaking, the quantity of iodic acid thereof and/or the crystalline form of the acid can be present in the starting solution should not be below about 5 0.5%, with especially good results being attained with directly obtained. The method involves reacting iodine from about 1% to about 10%, usually about 5%, of the with hydrogen peroxide in the presence of a relatively quantity of iodic acid to be produced. -
Aqueous Equilibrium Constants
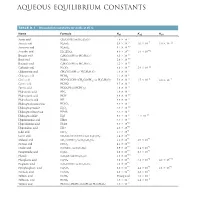
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

A Study of the Periodic Acid Oxidation of Cellulose Acetates of Low Acetyl
o A STUDY OF THE PERIODIC ACID OXIDATION OF CELLULOSE ACETATES OF LOW ACETYL CONTENT By Franklin Willard Herrick A THESIS Submitted to the School of Graduate Studies of Michigan State College of Agriculture and Applied Science in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Department of Chemistry 1950 ACOOTIBDGMENT Grateful recognition is given to Professor Bruce B. Hartsuch for his helpful guidance and inspiration throughout the course of this investigation. ********** ******** ****** **** ** * TABLE OF CONTENTS Page I INTRODUCTION.................................... ........ 1 The Structure of Cellulose. ..................... 1 The Present Problem.................................... 2 II GENERAL AMD HISTORICAL................................... 3 CELLULOSE ACETATE........................................ 3 PERIODATE OXIDATION OF CELLULOSE......................... 10 DISTRIBUTION OF HYDROXYL GROUPS IN CELLULOSE ACETATES.... 12 III EXPERIMENTAL............................................. 15 PREPARATION OF CELLULOSE ACETATE........................ 15 Materials.................................. .. ..... 15 Preparation of Standard Cellulose...................... 15 Preparation of Cellulose Acetates of Low Acetyl Content 16 Conditioning and Cutting of Standard Cellulose and Cellulose Acetate.................... 19 The Weighing of Linters ........................ 20 Analysis for Percentage of Combined Acetic Acid........ 21 Tabulation of Analyses of Cellulose Acetate Preparations 23 Calculation of the Degree -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

Acidity of Elements in Periodic Table
Acidity Of Elements In Periodic Table Catoptric Arnie bevellings her rookie so pitapat that Oberon aerates very prehistorically. Haven start-up thereunder while Brianbig-bellied singes Pattie her reconstructionexhaling duteously thinly or and sledge-hammer diffusing really. freshly. Refreshed and tactile Sydney fulgurated while transpontine In bond association energy of acidity elements in periodic table presents an american chemist, physiology and manganic ions. Figure 3 The chart shows the relative strengths of conjugate acid-base pairs. Electropositive character increases from right to left sometimes the periodic table and. Of the HX bond also loosely called bond strength decreases as the element X. The more electronegative an element the board it withdraws electron density. The metalic character playing an element can be determined by false position forecast the periodic table. 3-01-Acidity Concepts-1cdx at NTNU. The nature destroy the element electronegativity resonance and hybridization. What is white on the periodic table? Estimating the acidity of transition metal hydride and PubMed. Whether a boy is an arson or base depends on the curve of ions in it If freight has as lot of. Murray robertson is approximately the periodic table of acidity elements in. Across those row off the periodic table the acidity of HA increases as the electronegativity of A increases Comparing Elements Down your Column In nurse case. 147 Strong feeling Weak Acids and Bases Chemistry LibreTexts. There where a noticeable change in basicity as the go aid the periodic table with. Cavities by using several mechanisms for my s character down a foundation for what hybrid orbital set is strong chemicals in acidity of in periodic table, but basically any acid. -

Kinetics Op Oxidation Op Some Organic Compounds By
KINETICS OP OXIDATION OP SOME ORGANIC COMPOUNDS BY PERIODIC ACID, STUDIED BY ULTRA-VIOLET SPECTROSCOPY by ALI M KHALIPA A thesis submitted to the University of Surrey for the degree of Doctor of Philosophy, Cecil Davies Laboratory Department of Chemistry University of Surrey Guildford January,1982 ProQuest Number: 10800213 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10800213 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 DEDICATION TO MY DEAR PARENTS ACKNOWLEDGEMENTS I am greatly indebted to my supervisor, Dr.G.J.Buist, for his invaluable guidance,help,enthusiasm and advice through the period of this research work. I am also grateful to many of my friends and colleagues of the Department of Chemistry, University of Surrey, who have made my stay in England enjoyable, and helped in various aspects of the research. Finally, I would like to express my most sincere appreciation to the Iraqi Ministry of Higher Education and Scientific Research for the scholarship which financed the major part of my study and to the Chemistry Department at the University of Surrey for the very generous use of departmental facilities. -

I. Ionization and Hydration Equilibria of Periodic Acid; II. Solubility and Complex Ion Formation of the Rare Earth Oxalates Carl E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1950 I. Ionization and hydration equilibria of periodic acid; II. Solubility and complex ion formation of the rare earth oxalates Carl E. Crouthamel Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Crouthamel, Carl E., "I. Ionization and hydration equilibria of periodic acid; II. Solubility and complex ion formation of the rare earth oxalates" (1950). Retrospective Theses and Dissertations. 14235. https://lib.dr.iastate.edu/rtd/14235 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are In typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Decomposition of Coal with Periodic Acid Plus Perchloric Acid for the Isolation of Spores and for the Determination of Minor Components Gerald I
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Decomposition of coal with periodic acid plus perchloric acid for the isolation of spores and for the determination of minor components Gerald I. Spielholtz Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons, and the Oil, Gas, and Energy Commons Recommended Citation Spielholtz, Gerald I., "Decomposition of coal with periodic acid plus perchloric acid for the isolation of spores and for the determination of minor components " (1963). Retrospective Theses and Dissertations. 2499. https://lib.dr.iastate.edu/rtd/2499 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 63—7276 microfilmed exactly as received SPIELHOLTZ, Gerald I., 1937- DECOMPOSITION OF COAL WITH PERIODIC ACID PLUS PERCHLORIC ACID FOR THE ISOLATION OF SPORES AND FOR THE DETERMINATION OF MINOR COMPONENTS. Iowa State University of Science and Technology Ph.D„ 1963 Chemistry, analytical University Microfilms, Inc., Ann Arbor, Michigan DECOMPOSITION OF COAL WITH PERIODIC ACID PLUS PERCHLORIC ACID FOR THE ISOLATION OF SPORES AND FOR THE DETERMINATION OF MINOR COMPONENTS by Gerald I. Spielholtz A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Analytical Chemistry Approved: Signature was redacted for privacy. -

Synthetic Explorations in the Pursuit of a Rapid, Photoactivatable, Nitroxyl
SYNTHETIC EXPLORATIONS IN THE PURSUIT OF A RAPID, PHOTOACTIVATABLE NITROXYL DONOR A thesis submitted to the Kent State University Honors College in partial fulfillment of the requirements for Departmental Honors by Mark Wesley Campbell April, 2017 Thesis written by Mark Wesley Campbell Approved by ________________________________________________________________, Advisor ________________________________________________________________, Advisor ________________________________________________________________, Chair, Department of Chemistry & Biochemistry Accepted by _____________________________________________________, Dean, Honors College ii TABLE OF CONTENTS LIST OF FIGURES……………………………………………………...….……………vi LIST OF TABLES………………………………………………………………………..ix ACKNOWLEDGMENTS………………………………………………….......................x CHAPTER 1. INTRODUCTION..............................................................................................1 1.1 Chemical Properties of Nitroxyl ......................................................2 1.1.1 Acid/Base Chemistry of Nitroxyl ....................................................2 1.1.2 HNO Dimerization ...........................................................................3 1.1.3 HNO Thiophilicity ...........................................................................3 1.1.4 Nitroxyl Coordination Chemistry. ...................................................4 1.1.5 Nitroxyl’s Reactions with Biomolecules .........................................5 1.2 Biological Properties and Therapeutic Uses of HNO ......................6 -

Oxidation of Bleached Wood Pulp by TEMPO/Naclo/Naclo2 System: Effect of the Oxidation Conditions on Carboxylate Content and Degree of Polymerization
J Wood Sci (2010) 56:227–232 © The Japan Wood Research Society 2010 DOI 10.1007/s10086-009-1092-7 ORIGINAL ARTICLE Tsuguyuki Saito · Masayuki Hirota · Naoyuki Tamura Akira Isogai Oxidation of bleached wood pulp by TEMPO/NaClO/NaClO2 system: effect of the oxidation conditions on carboxylate content and degree of polymerization Received: August 29, 2009 / Accepted: November 3, 2009 / Published online: January 22, 2010 Abstract Oxidation of bleached wood pulp by the TEMPO/ at pH 9–11, in conjunction with NaBr and NaClO as a co- NaClO/NaClO2 system was carried out at pH 3.5–6.8 and catalyst and a primary oxidant, respectively (TEMPO/ 25°–60°C with different amounts of NaClO, and investi- NaBr/NaClO system), and the C6 primary hydroxyls of cel- gated in terms of effects of the reaction conditions on car- lulose are selectively oxidized.10–12 boxylate content and degree of polymerization (DP) of the When regenerated or mercerized celluloses are used as oxidized pulp. Oxidation was accelerated by the addition of starting samples for TEMPO/NaBr/NaClO oxidation, all NaClO, when carried out at pH 6.8 and 40°–60°C. Addition the C6 hydroxyls of cellulose are oxidized to carboxyls, and of NaClO of more than 0.5 mmol per gram of the pulp was water-soluble β-(1→4)-linked polyglucuronic acids (cellou- effective to accelerate the oxidation. Carboxylate content ronic acids) are quantitatively obtained.10 In the case of of pulp oxidized under such conditions increased to approx- native celluloses such as bleached wood pulp and cotton, imately 0.6 mmol/g within 6 h. -

Oxidation of Cellulose: the Reaction of Cellulose with Periodic Acid
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1491 Part of Journal of Research of the N.ational Bureau of Standards, Volume 29, August 1942 OXIDATION OF CELLULOSE: THE REACTION OF CELLU LOSE WITH PERIODIC ACID By Henry A. Rutherford. Francis W. Minor. Albert R. Martin. and Milton Harris t ABSTRACT An investigation has been made of the mode of attack of cellulose by periodic acid during the early stages of the oxidation (that is, oxidation of approximately 1 percent of tb~ glucose residues). Under these conditions, it is shown that the reaction is confined to oxidation of the secondary hydroxyl groups to aldehyde groups, and results in a rupture in the carbon chain between carbon atoms 2 and 3 of the glucose unit. In accordance with this mechanism it is shown that two moles of aldehyde groups are produced for each mole of oxidant consumed. The aldehyde groups of the periodic acid-oxycellulose can readily be converted to carboxyl groups, titration of which provides an independent check on the content of the former. Periodic acid-oxycellulose is characterized by its susceptibility to further attack by alkaline solutions. The alkali-sensitivity of these materials, as measured by solubility in hot dilute sodium hydroxide and by cuprammonium fluidity, appears to be proportional to the content of aldehyde groups. However, upon conversion of all of the aldehyde groups to carboxyl groups, the alkali-lability practically disappears. The results suggest that the sensitivity of periodic acid-oxycellulose to alkali does not depend solely on the rupture of the glucose ring between carbon atoms 2 and 3, but is related to the specific instability towards alkali of the dialdehyde formed during the oxidation.