Acid Nomenclature.Notebook February 02, 2017 Writing Acid Formulas/Naming Acids Formula Starts with "H" NameEnds in "Acid"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Group 17 (Halogens)
Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2020 Dr. Susan Findlay See Exercises 11.1 to 11.4 Forms of Carbon The Halogens (Group 17) What is a halogen? Any element in Group 17 (the only group containing Cl2 solids, liquids and gases at room temperature) Exists as diatomic molecules ( , , , ) Melting Boiling 2State2 2 2Density Point Point (at 20 °C) (at 20 °C) Fluorine -220 °C -188 °C Gas 0.0017 g/cm3 Chlorine -101 °C -34 °C Gas 0.0032 g/cm3 Br2 Bromine -7.25 °C 58.8 °C Liquid 3.123 g/cm3 Iodine 114 °C 185 °C Solid 4.93 g/cm3 A nonmetal I2 Volatile (evaporates easily) with corrosive fumes Does not occur in nature as a pure element. Electronegative; , and are strong acids; is one of the stronger weak acids 2 The Halogens (Group 17) What is a halogen? Only forms one monoatomic anion (-1) and no free cations Has seven valence electrons (valence electron configuration . ) and a large electron affinity 2 5 A good oxidizing agent (good at gaining electrons so that other elements can be oxidized) First Ionization Electron Affinity Standard Reduction Energy (kJ/mol) Potential (kJ/mol) (V = J/C) Fluorine 1681 328.0 +2.866 Chlorine 1251 349.0 +1.358 Bromine 1140 324.6 +1.065 Iodine 1008 295.2 +0.535 3 The Halogens (Group 17) Fluorine, chlorine and bromine are strong enough oxidizing agents that they can oxidize the oxygen in water! When fluorine is bubbled through water, hydrogen fluoride and oxygen gas are produced. -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

United States Patent Office
2,752,270 United States Patent Office Patented June 26, 1956 2 These impurities impair the crystallization of the solu 2,752,270 tions for the recovery of glucose; on the one hand, they PROCESS OF HYDROLYZNG wooD N PRE. increase the solubility of the glucose in the solvent, and PARNG CRYSTALLINE GLUCOSE on the other hand they retard the growth of the glucose Hugo Specht, Mannheim-Rheinau, Germany, assignor 5 crystals which remain so snail as to be very difficult to to Deutsche Bergin-Aktiengesellschaft, Mannheim separate from the syrup. I have found that crystalline Rheinau, Germany glucose can be obtained in good yields only when the No Drawing. Application January 5, 1954, Wood sugar Solution subjected to crystallization is so Serial No. 402,401 prepared as to consist essentially of glucose and to be free Claims priority, application Germany January 31, 1949 0 as far as possible from the recited impurities, including mannose. According to the invention, cellulosic ma 4 Claims. (C. 127-37) terial, for instance wood, is essentially freed from hemi The invention relates to improvements in the prepara celluloses; the residue should contain not more than tion of crystalline glucose by the hydrolysis of wood and about 5-6 per cent of sugars other than glucose, cal is a continuation-in-part of my copending application, 5 culated on the total content of carbohydrates, and is Serial No. 242,172, filed August 16, 1951, now aban then hydrolyzed with superconcentrated (39–42%) hy doned. drochloric acid. The thus obtained strongly acidic sugar Several processes have already been proposed for the solution is freed from hydrochloric acid by evaporation recovery of crystalline glucose from wood sugar solu So as to leave only a small residue of the acid; the mass tions, but they could not be economically operated on a 20 is then diluted to form a liquor containing about 10 to large scale. -

United States Patent Office Patented Apr
3,378,337 United States Patent Office Patented Apr. 16, 1968 1. 2 and the hydrogen peroxide. This result is achieved without 3,378,337 contaminating the reaction mixture. The final solution PREPARATHON OF ODEC ACID AND DERVATIVES THEREOF contains substantially pure iodic acid, and can be evap Ricardo O. Bach, Gastonia, N.C., assignor to Lithium orated and dehydrated to obtain the iodic acid, or the Corporation of America, Inc., New York, N.Y., a cor anhydride thereof, or it can be used as a medium for the poration of Minnesota direct production of salts of iodic acid. No Drawing. Fied May 17, 1965, Ser. No. 456,561 In carrying out the method of the present invention, 12 Claims. (CI. 23-85) the iodic acid solution employed in forming the reaction mixture should contain sufficient iodic acid to provide a O hydrogen ion and iodate ion concentration in the reaction ABSTRACT OF THE DISCLOSURE mixture which will favor oxidation of iodine to its penta A method of preparing solutions of substantially pure valent state and impede decomposition of hydrogen iodic acid from which salts of the acid, the anhydride peroxide. Generally speaking, the quantity of iodic acid thereof and/or the crystalline form of the acid can be present in the starting solution should not be below about 5 0.5%, with especially good results being attained with directly obtained. The method involves reacting iodine from about 1% to about 10%, usually about 5%, of the with hydrogen peroxide in the presence of a relatively quantity of iodic acid to be produced. -

Rate Constants for Reactions Between Iodine- and Chlorine-Containing Species: a Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction†
3708 J. Am. Chem. Soc. 1996, 118, 3708-3719 Rate Constants for Reactions between Iodine- and Chlorine-Containing Species: A Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction† Istva´n Lengyel,‡ Jing Li, Kenneth Kustin,* and Irving R. Epstein Contribution from the Department of Chemistry and Center for Complex Systems, Brandeis UniVersity, Waltham, Massachusetts 02254-9110 ReceiVed NoVember 27, 1995X Abstract: The chlorite-iodide reaction is unusual because it is substrate-inhibited and autocatalytic. Because - analytically pure ClO2 ion is not easily prepared, it was generated in situ from the rapid reaction between ClO2 and I-. The resulting overall reaction is multiphasic, consisting of four separable parts. Sequentially, beginning with mixing, these parts are the (a) chlorine dioxide-iodide, (b) chlorine(III)-iodide, (c) chlorine(III)-iodine, and (d) hypoiodous and iodous acid disproportionation reactions. The overall reaction has been studied experimentally and by computer simulation by breaking it down into a set of kinetically active subsystems and three rapidly established - equilibria: protonations of chlorite and HOI and formation of I3 . The subsystems whose kinetics and stoichiometries were experimentally measured, remeasured, or which were previously experimentally measured include oxidation of - iodine(-1,0,+1,+3) by chlorine(0,+1,+3), oxidation of I by HIO2, and disproportionation of HOI and HIO2. The final mechanism and rate constants of the overall reaction and of its subsystems were determined by sensitivity analysis and parameter fitting of differential equation systems. Rate constants determined for simpler reactions were fixed in the more complex systems. A 13-step model with the three above-mentioned rapid equilibria fits the - -3 - -3 - - overall reaction and all of its subsystems over the range [I ]0 < 10 M, [ClO2 ]0 < 10 M, [I ]0/[ClO2 ]0 ) 3-5, pH ) 1-3.5, and 25 °C. -
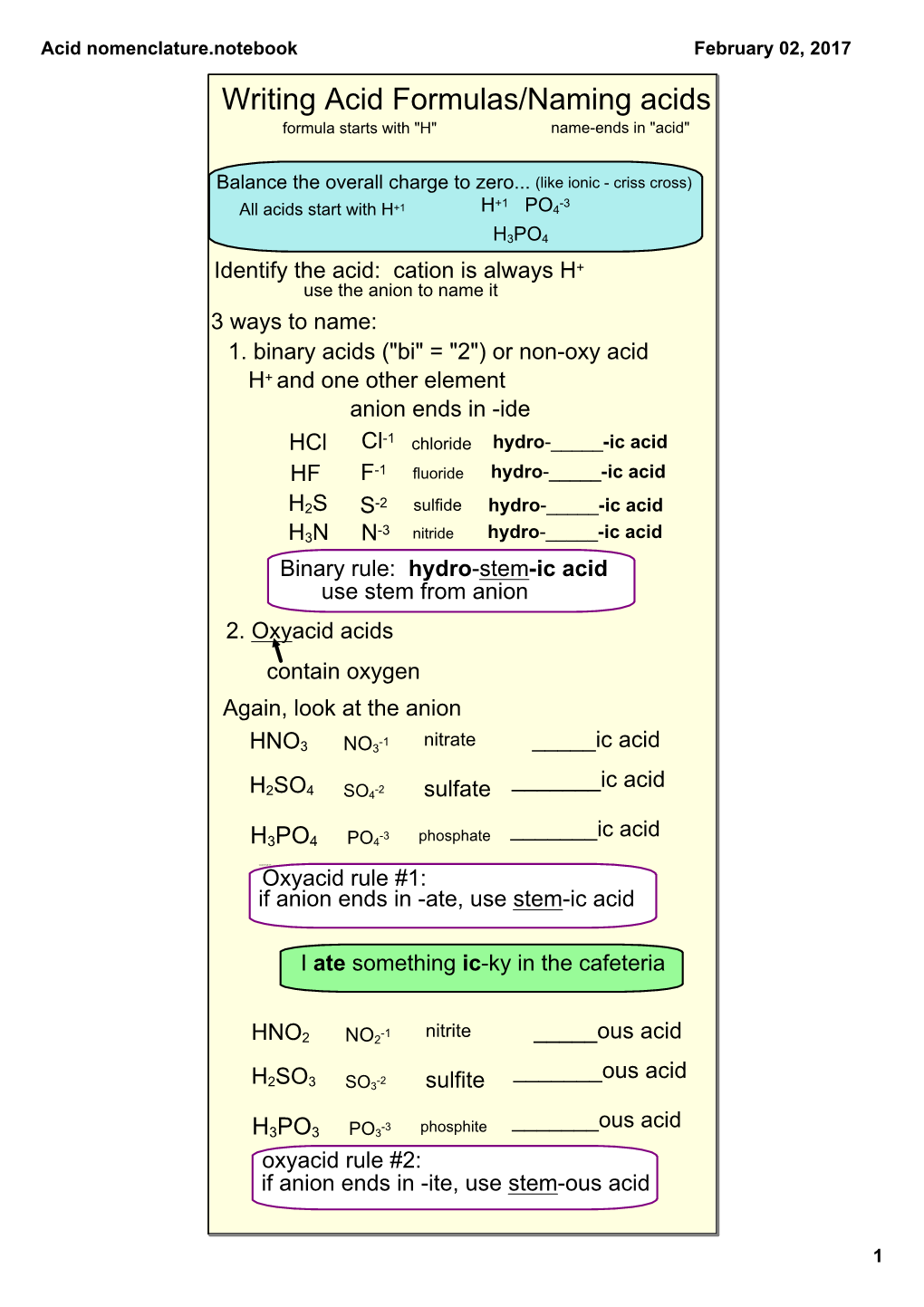
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -
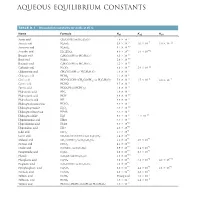
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

Guidance for Identification and Naming of Substance Under REACH
Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 GUIDANCE Guidance for identification and naming of substances under REACH and CLP May 2017 Version 2.1 2 Guidance for identification and naming of substances under REACH and CLP Version 2.1 - May 2017 LEGAL NOTICE This document aims to assist users in complying with their obligations under the REACH and CLP regulations. However, users are reminded that the text of the REACH and CLP Regulations is the only authentic legal reference and that the information in this document does not constitute legal advice. Usage of the information remains under the sole responsibility of the user. The European Chemicals Agency does not accept any liability with regard to the use that may be made of the information contained in this document. Guidance for identification and naming of substances under REACH and CLP Reference: ECHA-16-B-37.1-EN Cat. Number: ED-07-18-147-EN-N ISBN: 978-92-9495-711-5 DOI: 10.2823/538683 Publ.date: May 2017 Language: EN © European Chemicals Agency, 2017 If you have any comments in relation to this document please send them (indicating the document reference, issue date, chapter and/or page of the document to which your comment refers) using the Guidance feedback form. The feedback form can be accessed via the EVHA Guidance website or directly via the following link: https://comments.echa.europa.eu/comments_cms/FeedbackGuidance.aspx European Chemicals Agency Mailing address: P.O. Box 400, FI-00121 Helsinki, Finland Visiting address: Annankatu 18, Helsinki, Finland Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 PREFACE This document describes how to name and identify a substance under REACH and CLP. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

Dissociation Constants and Ph-Titration Curves at Constant Ionic Strength from Electrometric Titrations in Cells Without Liquid
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1537 Part of Journal of Research of the N.ational Bureau of Standards, Volume 30, May 1943 DISSOCIATION CONSTANTS AND pH-TITRATION CURVES AT CONSTANT IONIC STRENGTH FROM ELECTRO METRIC TITRATIONS IN CELLS WITHOUT LIQUID JUNCTION : TITRATIONS OF FORMIC ACID AND ACETIC ACID By Roger G. Bates, Gerda L. Siegel, and S. F. Acree ABSTRACT An improved method for obtaining the titration curves of monobasic acids is outlined. The sample, 0.005 mole of the sodium salt of the weak acid, is dissolver! in 100 ml of a 0.05-m solution of sodium chloride and titrated electrometrically with an acid-salt mixture in a hydrogen-silver-chloride cell without liquid junction. The acid-salt mixture has the composition: nitric acid, 0.1 m; pot assium nitrate, 0.05 m; sodium chloride, 0.05 m. The titration therefore is performed in a. medium of constant chloride concentration and of practically unchanging ionic strength (1'=0.1) . The calculations of pH values and of dissociation constants from the emf values are outlined. The tit ration curves and dissociation constants of formic acid and of acetic acid at 25 0 C were obtained by this method. The pK values (negative logarithms of the dissociation constants) were found to be 3.742 and 4. 754, respectively. CONTENTS Page I . Tntroduction __ _____ ~ __ _______ . ______ __ ______ ____ ________________ 347 II. Discussion of the titrat ion metbod __ __ ___ ______ _______ ______ ______ _ 348 1. Ti t;at~on. clU,:es at constant ionic strength from cells without ltqUld JunctlOlL - - - _ - __ _ - __ __ ____ ____ _____ __ _____ ____ __ _ 349 2.