Measurement of Iodine Species and Sulfuric Acid Using Bromide
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

United States Patent Office Patented Apr
3,378,337 United States Patent Office Patented Apr. 16, 1968 1. 2 and the hydrogen peroxide. This result is achieved without 3,378,337 contaminating the reaction mixture. The final solution PREPARATHON OF ODEC ACID AND DERVATIVES THEREOF contains substantially pure iodic acid, and can be evap Ricardo O. Bach, Gastonia, N.C., assignor to Lithium orated and dehydrated to obtain the iodic acid, or the Corporation of America, Inc., New York, N.Y., a cor anhydride thereof, or it can be used as a medium for the poration of Minnesota direct production of salts of iodic acid. No Drawing. Fied May 17, 1965, Ser. No. 456,561 In carrying out the method of the present invention, 12 Claims. (CI. 23-85) the iodic acid solution employed in forming the reaction mixture should contain sufficient iodic acid to provide a O hydrogen ion and iodate ion concentration in the reaction ABSTRACT OF THE DISCLOSURE mixture which will favor oxidation of iodine to its penta A method of preparing solutions of substantially pure valent state and impede decomposition of hydrogen iodic acid from which salts of the acid, the anhydride peroxide. Generally speaking, the quantity of iodic acid thereof and/or the crystalline form of the acid can be present in the starting solution should not be below about 5 0.5%, with especially good results being attained with directly obtained. The method involves reacting iodine from about 1% to about 10%, usually about 5%, of the with hydrogen peroxide in the presence of a relatively quantity of iodic acid to be produced. -

Iodine Oxoacids in Atmospheric Aerosol Formation – from Chamber Simulations to Field Observations
REPORT SERIES IN AEROSOL SCIENCE No. 239 (2021) IODINE OXOACIDS IN ATMOSPHERIC AEROSOL FORMATION – FROM CHAMBER SIMULATIONS TO FIELD OBSERVATIONS XU-CHENG HE (何旭成) Institute for Atmospheric and Earth System Research / Physics Faculty of Science, University of Helsinki, Helsinki, Finland Academic dissertation To be presented, with the permission of the Faculty of Science of the University of Helsinki, for public criticism in Exactum auditorium CK112, Pietari Kalmin Katu 5, on August 23rd, 2021, at 14 o'clock. Helsinki 2021 Author’s Address Institute for Atmospheric and Earth System Research / Physics P.O. box 64 FI-00140 University of Helsinki [email protected] Supervisors Academician, Professor Markku Kulmala, Ph.D. Institute for Atmospheric and Earth System Research / Physics University of Helsinki Associate Professor Mikko Sipilä, Ph.D. Institute for Atmospheric and Earth System Research / Physics University of Helsinki Assistant Professor Matti Petteri Rissanen, Ph.D. Aerosol Physics Laboratory Tampere University Lecturer Theo Kurtén, Ph.D. Department of Chemistry University of Helsinki Reviewers Research Professor Hannele Hakola, Ph.D. Air Quality Group Finnish Meteorological Institute Assistant Professor Julia Schmale, Ph.D. Extreme Environments Research Laboratory École polytechnique fédérale de Lausanne Opponent Professor Gordon McFiggans, Ph.D. Department of Earth and Environmental Sciences University of Manchester ISBN 978-952-7276-59-4 (printed version) ISSN 0784-3496 Helsinki 2021 Unigrafia Oy ISBN 978-952-7276-60-0 (pdf version) http://www.atm.helsinki.fi FAAR Helsinki 2021 Acknowledgements The work for this thesis was carried out at the Division of Atmospheric Sciences at the Department of Physics and later at the Institute for Atmospheric and Earth System Research (INAR), University of Helsinki. -

Rate Constants for Reactions Between Iodine- and Chlorine-Containing Species: a Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction†
3708 J. Am. Chem. Soc. 1996, 118, 3708-3719 Rate Constants for Reactions between Iodine- and Chlorine-Containing Species: A Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction† Istva´n Lengyel,‡ Jing Li, Kenneth Kustin,* and Irving R. Epstein Contribution from the Department of Chemistry and Center for Complex Systems, Brandeis UniVersity, Waltham, Massachusetts 02254-9110 ReceiVed NoVember 27, 1995X Abstract: The chlorite-iodide reaction is unusual because it is substrate-inhibited and autocatalytic. Because - analytically pure ClO2 ion is not easily prepared, it was generated in situ from the rapid reaction between ClO2 and I-. The resulting overall reaction is multiphasic, consisting of four separable parts. Sequentially, beginning with mixing, these parts are the (a) chlorine dioxide-iodide, (b) chlorine(III)-iodide, (c) chlorine(III)-iodine, and (d) hypoiodous and iodous acid disproportionation reactions. The overall reaction has been studied experimentally and by computer simulation by breaking it down into a set of kinetically active subsystems and three rapidly established - equilibria: protonations of chlorite and HOI and formation of I3 . The subsystems whose kinetics and stoichiometries were experimentally measured, remeasured, or which were previously experimentally measured include oxidation of - iodine(-1,0,+1,+3) by chlorine(0,+1,+3), oxidation of I by HIO2, and disproportionation of HOI and HIO2. The final mechanism and rate constants of the overall reaction and of its subsystems were determined by sensitivity analysis and parameter fitting of differential equation systems. Rate constants determined for simpler reactions were fixed in the more complex systems. A 13-step model with the three above-mentioned rapid equilibria fits the - -3 - -3 - - overall reaction and all of its subsystems over the range [I ]0 < 10 M, [ClO2 ]0 < 10 M, [I ]0/[ClO2 ]0 ) 3-5, pH ) 1-3.5, and 25 °C. -

Atmospheric Chemistry of Iodine ,† ,‡ § || § Alfonso Saiz-Lopez,* John† M
REVIEW pubs.acs.org/CR Atmospheric Chemistry of Iodine ,† ,‡ § || § Alfonso Saiz-Lopez,* John† M. C. Plane,* Alex^ R. Baker, Lucy J. Carpenter,‡ Roland von Glasow, Juan C. Gomez Martín, Gordon McFiggans, and Russell W. Saunders † Laboratory for Atmospheric and Climate Science (CIAC), CSIC, Toledo, Spain ‡ School of Chemistry, University of Leeds, Leeds, LS2 9JT, United Kingdom §School of Environmental Sciences, University of East Anglia, Norwich NR4 7TJ, United Kingdom Department) of Chemistry, University of York, Heslington, York YO10 5DD, United Kingdom ^ School of Earth, Atmospheric & Environmental Sciences, University of Manchester, Manchester, M13 9PL, United Kingdom CONTENTS 5.2.2. Particle Formation in Macroalgal 1. Introduction A Incubation Experiments R 2. Sources of Iodine to the Atmosphere C 5.2.3. Iodine Oxide Particle Growth to 2.1. Atmospheric Mixing Ratios, Seasonality, and Potential Cloud Condensation Nuclei S Trends in Distributions C 5.3. Unresolved Aspects of Iodine-Mediated Particle 2.2. Emission Rates and Mechanisms E formation S 2.3. Molecular Iodine H 6. Accumulation of Iodine in Aerosol U 2.4. Summary H 6.1. Iodine Concentrations in Aerosol U 3. Measurements of Gas-Phase Reactive Iodine Species I 6.2. Iodine Speciation U 3.1. Coastal Marine Boundary Layer I 6.3. Key Uncertainties V 7. Impacts and Modeling of Reactive Iodine Species V 3.2. Remote, Open Ocean, and Tropical Marine 7.1. Impacts of Iodine Chemistry V Boundary Layer I 7.1.1. Ozone Depletion V 3.3. Polar Boundary Layer J 7.1.2. Influence on the NO and HO Balance W 3.4. Salt Lakes and Volcanoes K x x 7.1.3. -

Phenol Disgrace Via Periodate in Integrating by Using Supersonic Radiation
Journal of Medicine and Life Vol. 8, Special Issue 3, 2015, pp.233-237 Phenol disgrace via Periodate in integrating by using Supersonic Radiation Seid-Mohamadi A*, Asgari G**, Shokoohi R**, Adabi S** *Social Determinants of Health Research Center, Department of Environmental Health Engineering, School of Public Health, Hamadan University of Medical Sciences, Hamedan, Iran, **Department of Environmental Health Engineering, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran Correspondence to: Shervin Adabi, MSc, Department of Environmental Health Engineering, School of Public Health, Hamadan University of Medical Sciences, Shahid Fahmideh Street, Hamadan, Iran Mobile phone: +989374196112, E-mail: [email protected] Received: March 14th, 2015 – Accepted: June 18th, 2015 Abstract In this study, a successful degradation of phenol was achieved by a combination of processes, ultrasonic irradiation and periodate. The effect of pH, dosage of IO4-, dosage of initial phenol and ultrasonic irradiation time on the phenol degradation were examined. Furthermore, the impacts of ion intensity on phenol degradation were examined. The findings indicated that the disgrace ratio advanced in acidic conditions and an upper degradation was achieved in combination processes. The current new investigation examined the effect of ion intensity and the findings determined that the principal intensity of solution is an inactive variable on phenol disgrace with these systems. A comparison research among IO4-/ US system and IO4- and US separately determined the COD removal and indicated that an combined method of IO4-/ US system had the best execution. Introduction degrade phenol residuals before the discharge to water sources. Advanced Oxidation Processes (AOPs) have Industries generate a considerable amount of been studied as efficient methods in last decades [5,6]. -
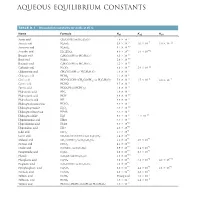
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Measurement Report: Indirect Evidence for the Controlling Influence of Acidity on the Speciation of Iodine in Atlantic Aerosols
Atmos. Chem. Phys., 21, 13067–13076, 2021 https://doi.org/10.5194/acp-21-13067-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Measurement report: Indirect evidence for the controlling influence of acidity on the speciation of iodine in Atlantic aerosols Alex R. Baker1 and Chan Yodle1,a 1Centre for Ocean and Atmospheric Sciences, School of Environmental Sciences, University of East Anglia, Norwich, NR4 7TJ, UK anow at: Department of Environmental Science, Faculty of Science and Technology, Chiang Mai Rajabhat University, Chiang Mai, 50300, Thailand Correspondence: Alex R. Baker ([email protected]) Received: 26 January 2021 – Discussion started: 26 April 2021 Revised: 22 July 2021 – Accepted: 10 August 2021 – Published: 2 September 2021 Abstract. The speciation of soluble iodine and major- 1 Introduction ion composition were determined in size-fractionated aerosols collected during the AMT21 cruise between Avon- mouth, UK, and Punta Arenas, Chile, in September– Iodine (I) plays a significant role in the destruction of ozone November 2011. The proportions of iodine species (iodide, (O3) in the atmosphere (Davis et al., 1996; Saiz-Lopez et iodate and soluble organic iodine (SOI)) varied markedly be- al., 2012), being responsible for ∼ 30 % of O3 loss in the tween size fractions and with the extent to which the samples marine boundary layer (MBL) (Prados-Roman et al., 2015). were influenced by pollutants. In general, fine mode aerosols Oceanic emission, principally of volatile I2 and hypoiodous (< 1 µm) contained higher proportions of both iodide and acid (HOI) formed through the reaction of O3 with iodide − SOI, while iodate was the dominant component of coarse (I ) at the sea surface (Carpenter et al., 2013), is the main (< 1 µm) aerosols. -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

Passivated Iodine Pentoxide Oxidizer for Potential Biocidal Nanoenergetic Applications Jingyu Feng, Guoqiang Jian, Qing Liu, and Michael R
Research Article www.acsami.org Passivated Iodine Pentoxide Oxidizer for Potential Biocidal Nanoenergetic Applications Jingyu Feng, Guoqiang Jian, Qing Liu, and Michael R. Zachariah* Department of Chemistry and Biochemistry, and Department of Chemical and Biomolecular Engineering, University of Maryland, College Park, Maryland 20742, United States *S Supporting Information ABSTRACT: Iodine pentoxide (I2O5), also known as diiodine pentoxide, is a strong oxidizer which has been recently proposed as an iodine-rich oxidizer in nanoenergetic formulations, whose combustion products lead to molecular iodine as a biocidal agent. However, its highly hygroscopic nature hinders its performance as a strong oxidizer and an iodine releasing agent and prevents its implementation. In this work, we developed a gas phase assisted aerosol spray pyrolysis which enables creation of iron oxide passivated I2O5. Transmission electron microscopy elemental imaging as well as temperature-jump mass spectrometry confirmed the core shell fi nature of the material and the fact that I2O5 could be encapsulated in pure unhydrated form. Combustion performance nds an optimal coating thickness that enables combustion performance similar to a high performing CuO based thermite. KEYWORDS: passivated, aerosol spray pyrolysis, nanothermite, energetic materials, nanocomposite, biocide 1. INTRODUCTION spores, presumably because of the release of elemental iodine as a combustion product when reacted with aluminum:16 10Al + Nanoenergetic materials, including metal based fuels and metal → 3I2O5 5Al2O3 +3I2. However, I2O5 is sensitive to humid oxide oxidizers with typically nanosized dimensions, have been 20,21 shown to have reactive properties superior to traditional environments and reacts with water in the ambient air. This 1−4 not only increases the particle size which degrades combustion energetic materials. -

United States Patent Office Patiented Sept
2,904,470 United States Patent Office Patiented Sept. 15, 1959 1 2 tion with bacteria or other organic matter, thus pro longing the activity of the solution. It is known that when elemental iodine is dissolved in 2,904,470 water a minute quantity of hypoiodous acid is formed. DIATOMICIODINE-HYPOIODOUS ACID GEN 5 However, this quantity of hypoiodous acid is too small to :ERATING COMPOSITIONS provide any useful therapeutic effect. It should be noted Abraham Berliner and Benjamin Carroll, New York, N.Y. that the solutions of the present invention contain hy poiodous acid at a concentration greater than that pro "No Drawing. Application December 1, 1955 duced when iodine is dissolved in water. Also the solu Serial No. 550,463 0 tions are preferably essentially free from iodide and tri iodide ions. 7 Claims, (Cl. 167-70) We have further found that aqueous solutions of diatomic iodine and hypoiodous acid are relatively un stable and that if effective germicidal action is to be This invention relates to germicides and more par 5 achieved the solutions must be prepared under carefully -ticularly to a novel iodine containing composition that controlled conditions as described herein. Because of the is adapted to be dissolved in water to produce an aqueous comparative instability of the solutions, it is desirable solution of diatomic iodine and hypoiodous acid, which that they be prepared shortly before they are used and solution is also new and possesses improved germicidal accordingly the present invention contemplates the use properties. Germicidal iodine-containing solutions made 20 of a solid composition which is readily soluble in water according to the present invention may be used for either and contains all the ingredients necessary to produce the therapeutic purposes or as sterilizing agents for a variety desired germicidal solution. -

ATOMIC ENERGY F F S L'energie ATOMIQUE of CANADA UMITED T
AECL-6f)2 ATOMIC ENERGY ffS L'ENERGIE ATOMIQUE OF CANADA UMITED Tif T DU CANADA UMITEE ASSESSMENT OF IODINE BEHAVIOUR IN REACTOR CONTAINMENT BUILDINGS FROM A CHEMICAL PERSPECTIVE EVALUATION DU COMPORTEMENT CHIMIQUE DE L'lODE DANS L'ENCEINTE DE PROTECTION D'UN REACTEUR Robert J. Lemire', Jean Paquette, David F. Torgerson , David J. Wren1, J. Wallace Fletcher2 Whiteshell Nuclear Research Establishment Etablissement de Recherches Nucle'aires de Whiteshell * Chalk River Nuclear Laboratories Laboratoires Nucleaires de Chalk River Whiteshell Nuclear Research Etablissement de Recherches Establishment Nucle'aires de Whiteshell Pinawa, Manitoba ROE 1L0 June T981 juin ATOMIC ENERGY OF CANADA LIMITED ASSESSMENT OF IODINE BEHAVIOUR IN REACTOR CONTAINMENT BUILDINGS FROM A CHEMICAL PERSPECTIVt by Robert J. Lemire , Jean Paquette , David F. Torgersor) , 1 2 David J. Wren and J. Wallace Fletcher Research Chemistry Branch Whiteshell Nuclear Research Establishment Physical Chemistry Branch Chalk River Nuclear Laboratories Whiteshell Nuclear Research Establishment Pinawa, Manitoba ROE 1L0 1981 June AECL-6812 EVALUATION DU COMPORTEMENT CHIMIQUE DE L'IODE DANS L'ENCEINTE DE PROTECTION D'UN REACTEUR par Robert J. Lemire , Jean Paquet te , David F. ïorgerson , 1 2 David J. Wren et J. Wallace Fletcher RESUME Les paramètres thermodynamiques pour les espèces aqueuses, solides et gazeuses de l'iode à 25°C ont été obtenus à partir d'un examen de la littérature chimique. Ces données ont été rendues com- patibles avec la compilation du groupe CODATA. En utilisant les données thermodynamiques à 25°C, les valeurs de l'énergie libre de formation ont été évaluées en fonction de la température, jusqu'à 150°C, et ce pour toutes les espèces de l'iode.