Dissociation Constants of Inorganic Acids and Bases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Group 17 (Halogens)
Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2020 Dr. Susan Findlay See Exercises 11.1 to 11.4 Forms of Carbon The Halogens (Group 17) What is a halogen? Any element in Group 17 (the only group containing Cl2 solids, liquids and gases at room temperature) Exists as diatomic molecules ( , , , ) Melting Boiling 2State2 2 2Density Point Point (at 20 °C) (at 20 °C) Fluorine -220 °C -188 °C Gas 0.0017 g/cm3 Chlorine -101 °C -34 °C Gas 0.0032 g/cm3 Br2 Bromine -7.25 °C 58.8 °C Liquid 3.123 g/cm3 Iodine 114 °C 185 °C Solid 4.93 g/cm3 A nonmetal I2 Volatile (evaporates easily) with corrosive fumes Does not occur in nature as a pure element. Electronegative; , and are strong acids; is one of the stronger weak acids 2 The Halogens (Group 17) What is a halogen? Only forms one monoatomic anion (-1) and no free cations Has seven valence electrons (valence electron configuration . ) and a large electron affinity 2 5 A good oxidizing agent (good at gaining electrons so that other elements can be oxidized) First Ionization Electron Affinity Standard Reduction Energy (kJ/mol) Potential (kJ/mol) (V = J/C) Fluorine 1681 328.0 +2.866 Chlorine 1251 349.0 +1.358 Bromine 1140 324.6 +1.065 Iodine 1008 295.2 +0.535 3 The Halogens (Group 17) Fluorine, chlorine and bromine are strong enough oxidizing agents that they can oxidize the oxygen in water! When fluorine is bubbled through water, hydrogen fluoride and oxygen gas are produced. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Download Download
Preparation of Telluric Acid from Tellurium Dioxide by Oxidation with Potassium Permanganate Frank C. Mathers, Charles M. Rice, Howard Broderick, and Robert Forney, Indiana University General Statement Tellurium dioxide, Te0 2 , although periodically similar to sulfur dioxide, cannot be oxidized by nitric acid to the valence of six, i.e., to telluric acid, H 2 Te04.2H 2 0. Among the many stronger oxidizing agents that will produce this oxidation, potassium permanganate in a nitric acid solution is quite satisfactory. This paper gives directions and data for the preparation of telluric acid by this reaction. The making of telluric acid is a desirable laboratory experiment because (1) tellurim dioxide is available in large quantities and is easily obtained, (2) the telluric acid is a stable compound, easily purified, easily crystallized, and non-corrosive, and (3) students are interested in experimenting with the rarer elements. The small soluibility of telluric acid and the high solubility of both manganese and potassium nitrates in nitric acidi gives a sufficient differ- ence in properties for successful purification by crystallization of the telluric acid. Methods of Analyses Tellurium dioxide can be volumetrically 2 titrated in a sulfuric acid solution by an excess of standard potassium permanganate, followed by enough standard oxalic acid to decolorize the excess of permanganate. The excess of oxalic acid must then be titrated by more of the perman- ganate. The telluric acid can be titrated, like any ordinary monobasic acid, 3 (1911). with standard sodium hydroxide using phenolphthalein as an indicator, if an equal volume of glycerine is added. If any nitric acid is present, it must be neutralized first with sodium hydroxide, using methyl orange as indicator. -

United States Patent Office Patented Apr
3,378,337 United States Patent Office Patented Apr. 16, 1968 1. 2 and the hydrogen peroxide. This result is achieved without 3,378,337 contaminating the reaction mixture. The final solution PREPARATHON OF ODEC ACID AND DERVATIVES THEREOF contains substantially pure iodic acid, and can be evap Ricardo O. Bach, Gastonia, N.C., assignor to Lithium orated and dehydrated to obtain the iodic acid, or the Corporation of America, Inc., New York, N.Y., a cor anhydride thereof, or it can be used as a medium for the poration of Minnesota direct production of salts of iodic acid. No Drawing. Fied May 17, 1965, Ser. No. 456,561 In carrying out the method of the present invention, 12 Claims. (CI. 23-85) the iodic acid solution employed in forming the reaction mixture should contain sufficient iodic acid to provide a O hydrogen ion and iodate ion concentration in the reaction ABSTRACT OF THE DISCLOSURE mixture which will favor oxidation of iodine to its penta A method of preparing solutions of substantially pure valent state and impede decomposition of hydrogen iodic acid from which salts of the acid, the anhydride peroxide. Generally speaking, the quantity of iodic acid thereof and/or the crystalline form of the acid can be present in the starting solution should not be below about 5 0.5%, with especially good results being attained with directly obtained. The method involves reacting iodine from about 1% to about 10%, usually about 5%, of the with hydrogen peroxide in the presence of a relatively quantity of iodic acid to be produced. -

DUDA ENERGY LLC Safety Data Sheet Urea (Prilled)
DUDA ENERGY LLC Safety Data Sheet Urea (Prilled) SECTION 1: Identification 1.1 Product identifier Product name Urea (Prilled) Product number Unavailable Brand Unavailable Substance name UREA EC no. 200-315-5 CAS no. 57-13-6 1.2 Other means of identification None 1.3 Recommended use of the chemical and restrictions on use Fertilizer, animal feed, plastics, chemical intermediate, stabilizer in explosives, medicine, adhesives, separation of hydrocarbons (as urea adducts), sulfamic acid production, flame proofing agents, viscosity modifier for starch, casein-based paper coatings, reported helpful in treatment of sickle-cell anemia, diuretic, and antiseptic. 1.4 Supplier's details Name Duda Energy LLC Address 1112 Brooks St. Decatur, AL 35601 USA Telephone 256.340.4866 Fax Unavailable email Unavailable 1.5 Emergency phone number(s) 800.255.3924 (Chemtel) SECTION 2: Hazard identification 2.1 Classification of the substance or mixture GHS classification in accordance with: UN GHS rev. 5 Not a hazardous substance or mixture. 2.2 GHS label elements, including precautionary statements Not a hazardous substance or mixture. 2.3 Other hazards which do not result in classification None known Statement regarding ingredients of unknown toxicity None SECTION 3: Composition/information on ingredients 3.1 Substances Substance name UREA EC no. 200-315-5 CAS no. 57-13-6 Formula CH4N2O Molecular weight 60.07 Other names / synonyms CARBAMIMIDIC ACID; UREA Impurities and stabilizing additives N/A SECTION 4: First-aid measures 4.1 Description of necessary first-aid measures General advice Ensure that medical personnel are aware of the material(s) involved and take precautions to protect themselves. -

1 the Volumetric Determination of Hydroxylamine
VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. I363 [CONTRIBUTION FROM THE CHEMICAL LABORATORYOF THE UNIVERSITY OF CALIFORNIA.1 THE VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. BY WILLIAMC. BRAY,MIBUM E. SIMPSONAND ANNA A. MACKENZIE. Received July 17, 1919 In the present investigation 3 volumetric methods of determining hydroxylamine in aqueous solution have been studied : The titanous salt method,' in which the hydroxylamine is reduced by excess titanous salt in acid solution with exclusion of air, and the excess titrated with permanganate. 2NH20H + Ti2(S04)3 = (NH4)2S04 + 4TiOS04 + HzS04. (I) The ferric salt method,2 in which the hydroxylamine is oxidized in an acid solution by excess of a ferric salt, the mixture is boiled and the fer- rous salt formed titrated with permanganate. 2NH20H + 2Fe@04)3 = N2O + 4FeS04 + 2H2S04 + H20. (2) The iodine method,3 in which the hydroxylamine is oxidized by iodine in a neutral solution, e. g., in the presence of disodium phosphate. 2NH20H + 212 = N2O + 4HI + H2O (3) or 2NH20H + 213- = N20 + 61- + 4H+ + HzO. Our first experiments, with the iodine method, yielded irregular results which could not be interpreted until the concentration of the hydroxyl- amine solution was accurately determined. An examination of the literature showed a rather unsatisfactory state of affairs. The advocates of the ferric sulfate method furnish evidence that it is perfectly reliable, but Leuba4 gives detailed experimental data to prove the contrary, and Adams5 states that he could not obtain reproducible results with it. The investigators who have used the iodine method consider it to be fairly satisfactory, but some of them state that it is not very accurate, and Rupp and Maeder6 have recently concluded that correct results are obtained only by a compensation of errors. -

Rate Constants for Reactions Between Iodine- and Chlorine-Containing Species: a Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction†
3708 J. Am. Chem. Soc. 1996, 118, 3708-3719 Rate Constants for Reactions between Iodine- and Chlorine-Containing Species: A Detailed Mechanism of the Chlorine Dioxide/Chlorite-Iodide Reaction† Istva´n Lengyel,‡ Jing Li, Kenneth Kustin,* and Irving R. Epstein Contribution from the Department of Chemistry and Center for Complex Systems, Brandeis UniVersity, Waltham, Massachusetts 02254-9110 ReceiVed NoVember 27, 1995X Abstract: The chlorite-iodide reaction is unusual because it is substrate-inhibited and autocatalytic. Because - analytically pure ClO2 ion is not easily prepared, it was generated in situ from the rapid reaction between ClO2 and I-. The resulting overall reaction is multiphasic, consisting of four separable parts. Sequentially, beginning with mixing, these parts are the (a) chlorine dioxide-iodide, (b) chlorine(III)-iodide, (c) chlorine(III)-iodine, and (d) hypoiodous and iodous acid disproportionation reactions. The overall reaction has been studied experimentally and by computer simulation by breaking it down into a set of kinetically active subsystems and three rapidly established - equilibria: protonations of chlorite and HOI and formation of I3 . The subsystems whose kinetics and stoichiometries were experimentally measured, remeasured, or which were previously experimentally measured include oxidation of - iodine(-1,0,+1,+3) by chlorine(0,+1,+3), oxidation of I by HIO2, and disproportionation of HOI and HIO2. The final mechanism and rate constants of the overall reaction and of its subsystems were determined by sensitivity analysis and parameter fitting of differential equation systems. Rate constants determined for simpler reactions were fixed in the more complex systems. A 13-step model with the three above-mentioned rapid equilibria fits the - -3 - -3 - - overall reaction and all of its subsystems over the range [I ]0 < 10 M, [ClO2 ]0 < 10 M, [I ]0/[ClO2 ]0 ) 3-5, pH ) 1-3.5, and 25 °C. -

A Fundamental Evaluation of the Atmospheric Pre-Leaching Section of the Nickel-Copper Matte Treatment Process
A FUNDAMENTAL EVALUATION OF THE ATMOSPHERIC PRE-LEACHING SECTION OF THE NICKEL-COPPER MATTE TREATMENT PROCESS by RODRICK MULENGA LAMYA Dissertation presented for the Degree of DOCTOR OF PHILOSOPHY (Extractive Metallurgical Engineering) in the Department of Process Engineering at the University of Stellenbosch, South Africa Promoter Prof. L. Lorenzen STELLENBOSCH March 2007 DECLARATION I the undersigned, hereby declare that the work contained in this dissertation is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. Signature: ............................................... Date: ....................................................... Copyright © 2007 Stellenbosch University All rights reserved i SYNOPSIS Nickel-Copper sulphide ores are the most important Platinum Group Metal bearing ores. The South African deposits are exceptionally rich in the platinum group metals (PGMs) and production of the PGMs is the primary purpose of treating these ores. The methods used in the recovery of the PGMs from the nickel-copper ores generally consists of ore concentration by physical techniques, pyrometallurgical concentration and hydrometallurgical extraction of the base metals followed by the PGMs. Pyrometallurgical concentration produces Ni-Cu matte, which is treated by hydrometallurgical processes to recover the nickel, copper, cobalt and the precious metals. In this study, the leaching behaviour of a Ni–Cu matte in CuSO4–H2SO4 solution during the repulping (pre-leach) stage at Impala Platinum Refineries was studied. The repulping stage is basically a non–oxidative atmospheric leach stage, in which nickel, iron and cobalt are partially dissolved, while the copper is precipitated. To understand the nature of the leaching process during this stage of the base metal refining operation, the effects of variations in the key process variables such as temperature, stirring rate, particle size, pulp density, residence time, initial copper and acid concentrations were investigated. -

Material Safety Data Sheet
Material Safety Data Sheet Issuing Date 2/25/2010 Revision Number 0 1. PRODUCT AND COMPANY IDENTIFICATION Product Name Sulfamic Acid Powder Product Code(s) 6286 Recommended Use Laboratory chemicals. Industrial (not for food or food contact use). Company LaMotte Company, Inc. 802 Washington Avenue P.O. Box 329 Chestertown, MD 21620 USA Emergency Telephone Number 24 Hour Emergency Number (CHEM-TEL): USA, Canada, Puerto Rico 1-800-255-3924 Outside North American Continent (Call collect) 813-248-0585 2. HAZARDS IDENTIFICATION DANGER! Emergency Overview Corrosive Causes SEVERE IRRITATION and BURNS to EVERY AREA of contact. Harmful or fatal if swallowed May cause lung damage Appearance White Physical State Crystalline Odor Odorless OSHA Regulatory Status This product is an article which contains a chemical substance. Safety information is given for exposure to the article as sold, but considers exposure to the chemical if user has direct eye and skin contact with the chemical. Potential Health Effects Principle Routes of Exposure Skin contact, Eye contact, Ingestion, and, Inhalation Acute Toxicity Eyes Causes burns. May cause irreversible damage to eyes. Skin Contact causes severe skin irritation and possible burns. Inhalation Inhalation of corrosive dusts can be extremely destructive to tissues of the mucous membranes and upper respiratory tract. Can cause burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting. May cause pulmonary edema, a medical emergency. Ingestion Ingestion causes burns of the upper digestive and respiratory tract. May be fatal if swallowed. Chronic Effects 3. COMPOSITION/INFORMATION ON INGREDIENTS Formula H2NSO3H Chemical Name CAS-No Weight % Sulfamic acid 5329-14-6 100 _____________________________________________________________________________________________ Published Date: 26-Feb-2010 Page 1 / 6 Sulfamic Acid Powder Product Code(s) 6286 _____________________________________________________________________________________________ 4. -
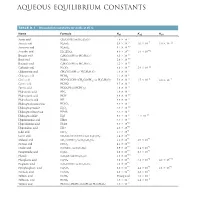
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

Guidance for Identification and Naming of Substance Under REACH
Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 GUIDANCE Guidance for identification and naming of substances under REACH and CLP May 2017 Version 2.1 2 Guidance for identification and naming of substances under REACH and CLP Version 2.1 - May 2017 LEGAL NOTICE This document aims to assist users in complying with their obligations under the REACH and CLP regulations. However, users are reminded that the text of the REACH and CLP Regulations is the only authentic legal reference and that the information in this document does not constitute legal advice. Usage of the information remains under the sole responsibility of the user. The European Chemicals Agency does not accept any liability with regard to the use that may be made of the information contained in this document. Guidance for identification and naming of substances under REACH and CLP Reference: ECHA-16-B-37.1-EN Cat. Number: ED-07-18-147-EN-N ISBN: 978-92-9495-711-5 DOI: 10.2823/538683 Publ.date: May 2017 Language: EN © European Chemicals Agency, 2017 If you have any comments in relation to this document please send them (indicating the document reference, issue date, chapter and/or page of the document to which your comment refers) using the Guidance feedback form. The feedback form can be accessed via the EVHA Guidance website or directly via the following link: https://comments.echa.europa.eu/comments_cms/FeedbackGuidance.aspx European Chemicals Agency Mailing address: P.O. Box 400, FI-00121 Helsinki, Finland Visiting address: Annankatu 18, Helsinki, Finland Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 PREFACE This document describes how to name and identify a substance under REACH and CLP. -

A Study of the Periodic Acid Oxidation of Cellulose Acetates of Low Acetyl
o A STUDY OF THE PERIODIC ACID OXIDATION OF CELLULOSE ACETATES OF LOW ACETYL CONTENT By Franklin Willard Herrick A THESIS Submitted to the School of Graduate Studies of Michigan State College of Agriculture and Applied Science in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Department of Chemistry 1950 ACOOTIBDGMENT Grateful recognition is given to Professor Bruce B. Hartsuch for his helpful guidance and inspiration throughout the course of this investigation. ********** ******** ****** **** ** * TABLE OF CONTENTS Page I INTRODUCTION.................................... ........ 1 The Structure of Cellulose. ..................... 1 The Present Problem.................................... 2 II GENERAL AMD HISTORICAL................................... 3 CELLULOSE ACETATE........................................ 3 PERIODATE OXIDATION OF CELLULOSE......................... 10 DISTRIBUTION OF HYDROXYL GROUPS IN CELLULOSE ACETATES.... 12 III EXPERIMENTAL............................................. 15 PREPARATION OF CELLULOSE ACETATE........................ 15 Materials.................................. .. ..... 15 Preparation of Standard Cellulose...................... 15 Preparation of Cellulose Acetates of Low Acetyl Content 16 Conditioning and Cutting of Standard Cellulose and Cellulose Acetate.................... 19 The Weighing of Linters ........................ 20 Analysis for Percentage of Combined Acetic Acid........ 21 Tabulation of Analyses of Cellulose Acetate Preparations 23 Calculation of the Degree