Geokniga-Metamorphic-Textures.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alignment Mouth Demonstrations in Sign Languages Donna Jo Napoli
Mouth corners in sign languages Alignment mouth demonstrations in sign languages Donna Jo Napoli, Swarthmore College, [email protected] Corresponding Author Ronice Quadros, Universidade Federal de Santa Catarina, [email protected] Christian Rathmann, Humboldt-Universität zu Berlin, [email protected] 1 Mouth corners in sign languages Alignment mouth demonstrations in sign languages Abstract: Non-manual articulations in sign languages range from being semantically impoverished to semantically rich, and from being independent of manual articulations to coordinated with them. But, while this range has been well noted, certain non-manuals remain understudied. Of particular interest to us are non-manual articulations coordinated with manual articulations, which, when considered in conjunction with those manual articulations, are semantically rich. In which ways can such different articulators coordinate and what is the linguistic effect or purpose of such coordination? Of the non-manual articulators, the mouth is articulatorily the most versatile. We therefore examined mouth articulations in a single narrative told in the sign languages of America, Brazil, and Germany. We observed optional articulations of the corners of the lips that align with manual articulations spatially and temporally in classifier constructions. The lips, thus, enhance the message by giving redundant information, which should be particularly useful in narratives for children. Examination of a single children’s narrative told in these same three sign languages plus six other sign languages yielded examples of one type of these optional alignment articulations, confirming our expectations. Our findings are coherent with linguistic findings regarding phonological enhancement and overspecification. Keywords: sign languages, non-manual articulation, mouth articulation, hand-mouth coordination 2 Mouth corners in sign languages Alignment mouth demonstration articulations in sign languages 1. -

Use of Theses
THESES SIS/LIBRARY TELEPHONE: +61 2 6125 4631 R.G. MENZIES LIBRARY BUILDING NO:2 FACSIMILE: +61 2 6125 4063 THE AUSTRALIAN NATIONAL UNIVERSITY EMAIL: [email protected] CANBERRA ACT 0200 AUSTRALIA USE OF THESES This copy is supplied for purposes of private study and research only. Passages from the thesis may not be copied or closely paraphrased without the written consent of the author. Language in a Fijian Village An Ethnolinguistic Study Annette Schmidt A thesis submitted for the degree of Doctor of Philosophy of the Australian National University. September 1988 ABSTRACT This thesis investigates sociolinguistic variation in the Fijian village of Waitabu. The aim is to investigate how particular uses, functions and varieties of language relate to social patterns and modes of interaction. ·The investigation focuses on the various ways of speaking which characterise the Waitabu repertoire, and attempts to explicate basic sociolinguistic principles and norms for contextually appropriate behaviour.The general purpose is to explicate what the outsider needs to know to communicate appropriately in Waitabu community. Chapter one discusses relevant literature and the theoretical perspective of the thesis. I also detail the fieldwork setting, problems and restrictions, and thesis plan. Chapter two provides the necessary background information to this study, describing the geographical, demographical and sociohistorical setting. Description is given of the contemporary language situation, structure of Fijian (Bouma dialect), and Waitabu social structure and organisation. In Chapter 3, the kinship system which lies at the heart of Waitabu social organisation, and kin-based sociolinguistic roles are analysed. This chapter gives detailed description of the kin categories and the established modes of sociolinguistic behaviour which are associated with various kin-based social identities. -

The Harrovian
THE HARROVIAN KING WILLIAM'S COLLEGE MAGAZINE Published three times yearly NUMBER 239 . DECEMBER THE BARRQVIAN 239 DECEMBER 1959 CONTENTS Random Notes School Officers Valete Salvete Library Notes Chapel Notes Correspondence Founder's Day Honours List University Admissions First House Plays ... Literary and Debating Society Literary Contributions Manx Society Gramophone Society Photographic Society Scientific Society Music Club The Orchestra Chess Notes Golf Society Aeronautical Society Badminton Society Shooting Combined Cadet Force ist K.W.C. Scout Group ... Swimming Cricket Rugby Football O.K.W. Section Obituaries Contemporaries We are grateful to the Isle of Man Times and Mona's Herald for permission to reprint photographs in this issue. THE BARROVIAN [December RANDOM NOTES Many friends, parents and O.K.W.'s will be interested to hear that Archdeacon Stenning in his capacity as Chaplain to the Royal Household has been asked to preach in the Chapel Royal on May 8th, 1960, at 10.45 a.m. * # * At the end of this term Mr. B. C. A. Hartley will be handing over as Housemaster of Junior House to Mr. C. Attwood. We should like to take this opportunity of joining the very large numbers of ex- Junior House boys and their parents who would like to thank Mr. Hartley for all the help and encouragement that he has given at Junior House for over twenty years. To say more at this stage would be to anticipate Mr. Hartley s retirement, an event happily many years distant. * * # We congratulate Dr. C. A. Caine (1942-49) who was elected into a Fellowship as Tutor in Mathematics at St. -
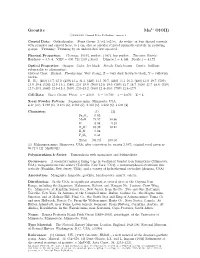
Groutite Mn3+O(OH) C 2001-2005 Mineral Data Publishing, Version 1
Groutite Mn3+O(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m2/m2/m. As wedge- or lens-shaped crystals with rounded and curved faces, to 1 cm; also as acicular striated prismatic crystals, in radiating groups. Twinning: Twinning by an undescribed law reported. Physical Properties: Cleavage: {010}, perfect; {100}, less perfect. Tenacity: Brittle. Hardness = 3.5–4 VHN = 630–711 (100 g load). D(meas.) = 4.144 D(calc.) = 4.172 Optical Properties: Opaque. Color: Jet-black. Streak: Dark brown. Luster: Brilliant submetallic to adamantine. Optical Class: Biaxial. Pleochroism: Very strong; X = very dark brown to black; Y = yellowish brown. R1–R2: (400) 13.7–22.0, (420) 13.4–21.3, (440) 13.3–20.7, (460) 13.1–20.2, (480) 13.0–19.7, (500) 13.0–19.4, (520) 12.9–19.1, (540) 12.9–19.0, (560) 12.8–19.0, (580) 12.7–18.7, (600) 12.7–18.6, (620) 12.7–18.5, (640) 12.6–18.3, (660) 12.5–18.2, (680) 12.4–18.0, (700) 12.4–17.9 Cell Data: Space Group: P bnm. a = 4.560 b = 10.700 c = 2.870 Z = 4 X-ray Powder Pattern: Sagamore mine, Minnesota, USA. 4.17 (10), 2.798 (6), 2.675 (6), 2.369 (6), 2.303 (5), 1.692 (5), 1.603 (4) Chemistry: (1) (2) Fe2O3 0.02 MnO 79.97 80.66 O 8.94 9.10 + H2O 10.39 10.24 − H2O 0.04 P2O5 0.34 Total [99.70] 100.00 (1) Mahnomen mine, Minnesota, USA; after correction for quartz 2.39%, original total given as 99.71% (2) MnO(OH). -

Dicionarioct.Pdf
McGraw-Hill Dictionary of Earth Science Second Edition McGraw-Hill New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2003 by The McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be repro- duced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-141798-2 The material in this eBook also appears in the print version of this title: 0-07-141045-7 All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. (“McGraw- Hill”) and its licensors reserve all rights in and to the work. Use of this work is subject to these terms. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decom- pile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, distribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hill’s prior consent. -

Geology of the Pegmatites and Associated Rocks of Maine
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR BULLETIN 445 GEOLOGY OF THE PEGMATITES AND ASSOCIATED ROCKS OF MAINE INCLUDING FELDSPAR, QUARTZ, MICA, AND GEM DEPOSITS BY EDSON S. BASTIN WASHINGTON GOVERNMENT PRINTING OFFICE 1911 CONTENTS. Introduction.............................................................. 9 Definition of pegmatite...................................................... 10 Geographic distribution.................................................... 10 Geology.................................................................. 10 Bordering rocks....................................................... 10 Pegmatites in foliated rocks........................................ 11 General statement............................................ 11 Sedimentary foliates........................................... 11 Igneous foliates.....".......................................... 12 Pegmatites in massive granites.................................... 13 'Age.................................................................. 15 General character..................................................... 15 Mineral and chemical composition................................. 15 Mineral constituents.......................................... 15 Relative proportions of minerals............................... 18 Quartzose phases. ..............................^............. 18 Fluidal cavities............................................... 19 Sodium and lithium phases................................... 20 Muscovite -

Out-Of-School Children: a Contemporary View from the Pacific Island Countries of the Commonwealth
C O L C O L Out-of-School Children: A Contemporary View from the Pacific Island Countries of the Commonwealth Sharishna Narayan With support from Tony Mays Som Naidu Kirk Perris Out-of-School Children: A Contemporary View from the Pacific Island Countries of the Commonwealth Sharishna Narayan With support from Tony Mays Som Naidu Kirk Perris The Commonwealth of Learning (COL) is an intergovernmental organisation created by Commonwealth Heads of Government to encourage the development and sharing of knowledge, resources and technologies in open learning and distance education. Commonwealth of Learning, 2021 © 2021 by the Commonwealth of Learning. Out-of-School Children: A Contemporary View from the Pacific Island Countries of the Commonwealth is made available under a Creative Commons Attribution-ShareAlike 4.0 International Licence, https://creativecommons.org/licenses/by-sa/4.0/. For avoidance of doubt, by applying this licence, the Commonwealth of Learning does not waive any privileges or immunities from claims that they may be entitled to assert, nor does the Commonwealth of Learning submit itself to the jurisdiction, courts, legal processes or laws of any jurisdiction. ISBN: 978-1-7772648-4-0 Cover photo: M M from Switzerland, CC BY-SA 2.0 <https://creativecommons.org/ licenses/by-sa/2.0>, via Wikimedia Commons Published by: COMMONWEALTH OF LEARNING 4710 Kingsway, Suite 2500 Burnaby, British Columbia Canada V5H 4M2 Telephone: +1 604 775 8200 Fax: +1 604 775 8210 Web: www.col.org Email: [email protected] Contents Acknowledgements ................................................................................ viii Acronyms and Abbreviations ................................................................... ix 1. Overview ..................................................................................................... 1 Exploratory Study of Out-of-School Children (OOSC) in the Pacific: 2. -

THE CONSTITUTION (AMENDMENT) BILL, 2015 By
AS INTRODUCED IN LOK SABHA Bill No. 90 of 2015 THE CONSTITUTION (AMENDMENT) BILL, 2015 By SHRI ASHWINI KUMAR CHOUBEY, M.P. A BILL further to amend the Constitution of India. BE it enacted by Parliament in the Sixty-sixth Year of the Republic of India as follows:— 1. This Act may be called the Constitution (Amendment) Act, 2015. Short title. 2. In the Eighth Schedule to the Constitution,— Amendment of the Eighth (i) existing entries 1 and 2 shall be renumbered as entries 2 and 3, respectively, Schedule. 5 and before entry 2 as so renumbered, the following entry shall be inserted, namely:— "1. Angika."; (ii) after entry 3 as so renumbered, the following entry shall be inserted, namely:— "4. Bhojpuri."; and (iii) entries 3 to 22 shall be renumbered as entries 5 to 24, respectively. STATEMENT OF OBJECTS AND REASONS Language is not only a medium of communication but also a sign of respect. Language also reflects on the history, culture, people, system of governance, ecology, politics, etc. 'Bhojpuri' language is also known as Bhozpuri, Bihari, Deswali and Khotla and is a member of the Bihari group of the Indo-Aryan branch of the Indo-European language family and is closely related to Magahi and Maithili languages. Bhojpuri language is spoken in many parts of north-central and eastern regions of this country. It is particularly spoken in the western part of the State of Bihar, north-western part of Jharkhand state and the Purvanchal region of Uttar Pradesh State. Bhojpuri language is spoken by over forty million people in the country. -

GUIDE MINERALS and ROCKS
GUIDE to the MINERALS and ROCKS of Minnesota by GEORGE M. SCHWARTZ and GEORGE A. THIEL DEPARTMENT OF GEOLOGY and MINNESOTA GEOLOGICAL SURVEY UNIVERSITY OF MINNESOTA Guide to the Minerals and Rocks of Minnesota by G. M. Schwartz, Director, Minnesota Geological Survey George A. Thiel, Chairman, Department of Geology University of Minnesota Introduction The minerals and rocks of Minnesota serve as the basis of many im portant industries. They supply the parent materials for our many different types of soils; they contain our vital underground water supplies; and they furnish the raw materials for extensive mineral industries. Many people are interested in the rocks and minerals which they find in road-cuts, in excavations, on their farms, and on their vacation trips. Unusual specimens arouse the curiosity of the finder. Some specimens have peculiar shapes, some have attractive colors and others appear to contain ores of economic value: Many such specimens are received each year at the Department of Geology and the Minnesota Geological Survey of the University of Minnesota. The specimens are generally accompanied by a request for information in regard to the composition, manner of formation, and commercial value of the rock or mineral involved. In response to an increasing number of inquiries from individuals and from schools, this pamphlet was prepared to summarize, as far as possible in nontechnical terms, the different kinds of rocks and minerals found in Minnesota and to describe them so that the amateur collector, the school teacher, the boy and girl scout and other interested persons can identify them. In a pamphlet of this size it is impossible to outline in detail the almost infinite varieties of the common rocks and minerals. -

Mineralogy of the Peraluminous Spruce Pine Plutonic Suite
MINERALOGY OF THE PERALUMINOUS SPRUCE PINE PLUTONIC SUITE, MITCHELL, AVERY, AND YANCEY COUNTIES, NORTH CAROLINA by William Brian Veal (Under the Direction of Samuel E. Swanson) ABSTRACT The Spruce Pine plutonic suite consists of numerous Devonian (390-410 ma) peraluminous granitic plutons, dikes, sills, and associated pegmatites. The mineralogy of pegmatites and host granodiorites from seven locations was studied to determine the extent of fractionation of the pegmatites relative to the granodiorite. Garnets in the plutons are almandine- spessartine and some display epitaxial overgrowths of grossular garnet. Similar garnets are found in the pegmatites. Pegmatitic muscovite contains several percent iron and is compositionally similar to muscovite from the host granodiorite. Feldspars in the granodiorites and pegmatites are compositionally similar (plagioclase Ab69-98; k-feldspar Or95-98). The lack of compositional variation between minerals in pegmatites and host granodiorites indicates the pegmatites were not the result of extreme fractionation and indicates that pegmatite mineralogy can be used to characterize the mineralogy of an entire granodiorite body. INDEX WORDS: Spruce Pine, Pegmatites, Granodiorite, Garnet, Muscovite, Feldspar MINERALOGY OF THE PERALUMINOUS SPRUCE PINE PLUTONIC SUITE, MITCHELL, AVERY, AND YANCEY COUNTIES, NORTH CAROLINA by WILLIAM BRIAN VEAL B.S., Georgia Southwestern State University, 2002 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2004 © 2004 William Brian Veal All Rights Reserved MINERALOGY OF THE PERALUMINOUS SPRUCE PINE PLUTONIC SUITE, MITCHELL, AVERY, AND YANCEY COUNTIES, NORTH CAROLINA by WILLIAM BRIAN VEAL Major Professor: Samual E. Swanson Committee: Michael F. -

Textures and Structures of Igneous Rocks
UNIT 2 TEXTURES AND STRUCTURES OF IGNEOUS ROCKS Structure______________________________________________ 2.1 Introduction 2.4 Forms of Igneous Rocks Expected Learning Outcomes Sill 2.2 Textures of Igneous Rocks Dyke Crystallinity Laccolith Granularity Bysmalith Shape of the Crystals Lopolith Mutual Relationship between Crystal and Phacolith Non-Crystalline Material Chonolith Intergrowth Textures Volcanic Neck Exsolution Textures Batholith Miscellaneous Textures Stock 2.3 Structures of Igneous Rocks Boss Vesicular and Amygdaloidal Structures 2.5 Summary Scoriaceous and Pumiceous Structures 2.6 Activity Lava Tunnels 2.7 Terminal Questions Blocky and Ropy Lava 2.8 References Platy and Sheet Structure 2.9 Further/Suggested Readings Pillow Lava 2.10 Answers Columnar/Prismatic Structure Lava Flow Structure Rift and Grain Perlitic Structure Rapakivi Structure Xenoliths ……………………………………………………………………………………………….…....Block 1 Igneous Petrology.......…-I 2.1 INTRODUCTION You have been introduced to the igneous rocks in the previous unit. You have also learnt that the slow cooling of magma takes place in the deeper parts of the Earth and large size crystals are formed. On the contrary, magma undergoing rapid cooling in shallower depth or on the surface of the Earth yielded fine grained crystals. The rapid cooling of lava/melt molten rock on the surface of the Earth produces fine grained minerals or glass. The igneous rocks vary in grain size from very coarse, medium to fine grained or even glassy in hand specimen and under the microscope. Thus, igneous rocks display variety of textures. Thus, the term texture refers to physical appearance of a rock. In this unit we will discuss textures and structures of igneous rocks. -

Subsurface Geology of the Cochran Pegmatite, Cherokee-P…
SUBSURFACE GEOLOGY OF THE COCHRAN PEGMATITE, CHEROKEE-PICKENS DISTRICT, GEORGIA Alexander J. Gunow Gregory N. Bonn Bruce J. O'Connor Georgia Department of Natural Resources Environmental Protection Division Georgia Geologic Survey GEOLOGIC REPORT 5 Subsurface Geology of the Cochran Pegmatite, Cherokee-Pickens District, Georgia Alexander J. Gunow Gregory N. Bonn Bruce J. O'Connor Georgia Department of Natural Resources Joe D. Tanner, Commissioner Environmental Protection Division Harold F. Reheis, Director Georgia Geologic Survey William H. McLemore, State Geologist Atlanta 1992 Geologic Report 5 TABLE OF CONTENTS Page Abstract ..................................................................................................... 1 Introduction ............................................................................................. 2 General r>escription ................................................................................ 3 Results ....................................................................................................... 6 Discussion................................................................................................. 9 References ................................................................................................. 10 Appendices ........................................................................................... .... 11 A. Drill Hole Information and Mineralogy ......................... 11 B. Lithologic r>escription of Drill Core ............................... 17 LIST OF ILLUSTRATIONS