GUIDE MINERALS and ROCKS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Devonian and Carboniferous Metamorphism in West-Central
American Mineralogist, Volume 73, pages20-47, 1988 Devonian and Carboniferousmetamorphism in west-centralMaine: The muscovite-almandinegeobarometer and the staurolite problem revisited M. J. Hololwlv Department of Geological Sciences,Southern Methodist University, Dallas, Texas 75275, U.S.A. B. L. Durnow Department of Geology, Louisiana State University, Baton Rouge, Louisiana 70803, U.S.A. R. W. HrNroN* The EnricoFermi Institute, The Universityof Chicago,Chicago, Illinois 60637,U.S.A. ABSTRAcT Important thermal metamorphic eventsin west-centralMaine occurred at 400 Ma (Mr), 394-379Ma(Mr), and325 Ma(Mr). EachiscloselyassociatedwithemplacementofS-type granites,such that the isogradpatterns produced in the surrounding pelitic schistsgenerally follow plutonic outlines. From north to south, gradeof metamorphism varies from chlorite to sillimanite-K-feldspar-muscovite. Mineral-chemistry studies on M, and M, indicate the following: (l) Much staurolite- grade chlorite is retrograde as demonstrated by the lack of a consistent relation between biotite composition and presenceor absenceofchlorite, given the restricted range ofX"ro allowed in these reduced graphitic rocks. Consequently,the first sillimanite-forming re- action for many pelitic schists in Maine does not involve chlorite. (2) Garnet zoning patterns are prograde in rocks of the staurolite zone and retrograde in rocks of higher grade. (3) Presenceof graphite and nearly pure ilmenite suggestslow Fe3* in micas and allows for end-membercalculations that include a "Ti biotite" (KTiD(Fe,Mg)AlSi3Oro(OH)r) and a "Ti muscovite" (KTi(Fe,Mg)AlSi3O,o(OH)r).(4) Staurolire contains about 3 H (48- oxygen basis) and shows subtle indications of nonideality in Fe-Mg Ko relationships. Garnet-biotite geothermometryindicates the following averagetemperatures for the first occurrenceof minerals:staurolite-510'C, sillimanite-580 "C, sillimanite-K-feldspar- 660 "C. -

Miissbauer Spectra of Minnesotaite and Ferroan Talc J.M.D. Corv, T
American Mineralogist, Volume 76, pages 1905-1909, 1991 Miissbauer spectra of minnesotaite and ferroan talc J.M.D. Corv, T. Blxls* Department of Pure and Applied Physics,Trinity College,Dublin 2, Ireland S. GuccnNnprnr Department of Geological Sciences,University of Illinois at Chicago, Chicago,Illinois 60680, U.S.A, Arsrru.cr Mdssbauer spectra of five samples of minnesotaite and one of ferroan talc have been recordedat 296 K and 4.2 K. A singleferrous doublet with isomer shift 6 : 1.13 mm/s relative to aFe and quadrupolesplitting L:2J4 mm/s is observedin all minnesotaite samples at room temperature. The ferroan talc has a smaller quadrupole splitting, A : 2.55 mm/s. All minnesotaite samplesorder antiferromagnetically,with N6el points in the tange 25-37 K, but ferroan talc does not order magnetically down to 4.2 K. Two distinct ferrous populations in minnesotaite are inferred from the resolved magneticallysplit spec- tra at 4.2 K. The minority sites may be those coordinated by the excessOH in the min- nesotaitestructure. The P-cell and C-cell variants have distinctly different hyperfine fields (10.4 and 14.9T for the two sitesin the P cell but 8.9 and 14.4T in the C cell),although their N6el points are similar. INrnooucrroN taite, ferroan talc, and talc and found a lack of compo- Minnesotaite was originally considered by Gruner sitions having the octahedralFe fraction in the range0.45- (1944) to be an Fe analogueof talc having ideal formula 0.60, perhapsindicating the chemical limits of the struc- tures. {Fel*}[Sio]O,o(OH),,where { } and [ ] denotethe oc- tahedral and tetrahedral sites, respectively.More recent- Both minnesotaite and talc have a continuous octahe- ly, X-ray and electron diffraction studies (Guggenheim dral sheet(Fe in minnesotaite, Fe,Mg in ferroan talc) but and Bailey, 1982; Guggenheim and Eggleton, 1986) differ in the nature ofthe tetrahedral sheets.It is therefore showed that the structure is considerablymore complex. -
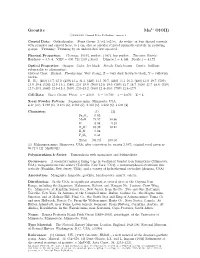
Groutite Mn3+O(OH) C 2001-2005 Mineral Data Publishing, Version 1
Groutite Mn3+O(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m2/m2/m. As wedge- or lens-shaped crystals with rounded and curved faces, to 1 cm; also as acicular striated prismatic crystals, in radiating groups. Twinning: Twinning by an undescribed law reported. Physical Properties: Cleavage: {010}, perfect; {100}, less perfect. Tenacity: Brittle. Hardness = 3.5–4 VHN = 630–711 (100 g load). D(meas.) = 4.144 D(calc.) = 4.172 Optical Properties: Opaque. Color: Jet-black. Streak: Dark brown. Luster: Brilliant submetallic to adamantine. Optical Class: Biaxial. Pleochroism: Very strong; X = very dark brown to black; Y = yellowish brown. R1–R2: (400) 13.7–22.0, (420) 13.4–21.3, (440) 13.3–20.7, (460) 13.1–20.2, (480) 13.0–19.7, (500) 13.0–19.4, (520) 12.9–19.1, (540) 12.9–19.0, (560) 12.8–19.0, (580) 12.7–18.7, (600) 12.7–18.6, (620) 12.7–18.5, (640) 12.6–18.3, (660) 12.5–18.2, (680) 12.4–18.0, (700) 12.4–17.9 Cell Data: Space Group: P bnm. a = 4.560 b = 10.700 c = 2.870 Z = 4 X-ray Powder Pattern: Sagamore mine, Minnesota, USA. 4.17 (10), 2.798 (6), 2.675 (6), 2.369 (6), 2.303 (5), 1.692 (5), 1.603 (4) Chemistry: (1) (2) Fe2O3 0.02 MnO 79.97 80.66 O 8.94 9.10 + H2O 10.39 10.24 − H2O 0.04 P2O5 0.34 Total [99.70] 100.00 (1) Mahnomen mine, Minnesota, USA; after correction for quartz 2.39%, original total given as 99.71% (2) MnO(OH). -

Greenalite, Mg-Rich Minnesotaite and Stilpnomelane from the Osj6berg and Sirsj6berg Iron-Ore Mines, Hjulsj6, W
MINERALOGICAL MAGAZINE, SEPTEMBER 1985, VOL. 49, PP. 611 613 Greenalite, Mg-rich minnesotaite and stilpnomelane from the Osj6berg and Sirsj6berg iron-ore mines, Hjulsj6, W. Bergslagen, Sweden GREENALITE, Mg-rich minnesotaite, and stilpno- subvertical body which strikes approximately E-W melane have been identified by electron micro- and is considered by Baker and de Groot (1983b) to probe analysis in samples taken from dumps of the be continuation of the Osj6berg ore horizon. The abandoned Qsj6berg and Sirsj6berg mines 5 km ore is separated from an underlying limestone NW of Hjulsj6, Bergslagen. This is the first horizon by a garnet-pyroxene-amphibole skarn. report of the occurrence of these minerals in the Magnetite is present in the skarn and the limestone. Proterozoic iron ores of Central Sweden. The two Epidote and calcite are later minerals filling tectonic mines, some 700 m apart, worked a large, 7 km long, fissures and cracks. meta-rhyotite-hosted, magnetite ore horizon--one Greenalite occurs in a sample from ()sj6berg of the many concordant skarn iron ores in the mine which comprises a fine grained aggregate of 1.8-1.9 Ga Proterozoic Bergslagen Supracrustal magnetite grains (+0.05 mm) with some larger Series (Oen et al., 1982). They were amongst the grains up to 0.5 mm in a matrix composed pre- largest iron producers of this area. dominantly of talc with minor green chlorite. Talc The subsurface geology of the mines has been also fills fractures in the larger magnetite grains. described by Geijer and Magnusson (1944); earlier Very minor pyrite and rare chalcopyrite are seen descriptions are given by Blomberg (1879) and enclosing magnetite. -

Data of Geochemistry
Data of Geochemistry ' * Chapter W. Chemistry of the Iron-rich Sedimentary Rocks GEOLOGICAL SURVEY PROFESSIONAL PAPER 440-W Data of Geochemistry MICHAEL FLEISCHER, Technical Editor Chapter W. Chemistry of the Iron-rich Sedimentary Rocks By HAROLD L. JAMES GEOLOGICAL SURVEY PROFESSIONAL PAPER 440-W Chemical composition and occurrence of iron-bearing minerals of sedimentary rocks, and composition, distribution, and geochemistry of ironstones and iron-formations UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1966 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 45 cents (paper cover) CONTENTS Page Face Abstract. _ _______________________________ Wl Chemistry of iron-rich rocks, etc. Continued Introduction. _________ ___________________ 1 Oxide facies Continued Iron minerals of sedimentary rocks __ ______ 2 Hematitic iron-formation of Precambrian age__ W18 Iron oxides __ _______________________ 2 Magnetite-rich rocks of Mesozoic and Paleozoic Goethite (a-FeO (OH) ) and limonite _ 2 age___________-__-._____________ 19 Lepidocrocite (y-FeO(OH) )________ 3 Magnetite-rich iron-formation of Precambrian Hematite (a-Fe2O3) _ _ _ __ ___. _ _ 3 age._____-__---____--_---_-------------_ 21 Maghemite (7-Fe203) __ __________ 3 Silicate facies_________________________________ 21 Magnetite (Fe3O4) ________ _ ___ 3 Chamositic ironstone____--_-_-__----_-_---_- 21 3 Silicate iron-formation of Precambrian age_____ 22 Iron silicates 4 Glauconitic rocks__-_-____--------__-------- 23 4 Carbonate facies______-_-_-___-------_---------- 23 Greenalite. ________________________________ 6 Sideritic rocks of post-Precambrian age._______ 24 Glauconite____ _____________________________ 6 Sideritic iron-formation of Precambrian age____ 24 Chlorite (excluding chamosite) _______________ 7 Sulfide facies___________________________ 25 Minnesotaite. -

Petrology of the Low-Grade Rocks of the Gunflint Iron-Formation, Ontario-Minnesota
Petrology of the Low-Grade Rocks of the Gunflint Iron-Formation, Ontario-Minnesota PAP?k^N I Department of Earth and Space Sciences, State University of New York, Stony Brook, New York 11794 ABSTRACT tent with textural and compositional data tinuation of the iron formation. Marsden supporting a primary origin for the iron and others (1968) used the term "Animikie The relatively unmetamorphosed middle silicates. Quartz, recrystallized carbonate Iron Formation" for the correlated seg- Precambrian Gunflint Iron-Formation of cements, microcrystalline siderite, hematite, ments of the Cuyuna, Mesabi, and Gunflint Ontario has undergone considerable post- and possibly magnetite are also considered Ranges of Minnesota and Ontario. depositional recrystallizarion and locally in- primary phases. Key words: mineralogy, The iron formation is structurally simple tense replacement. Although these tend to sedimentary petrology, crystal chemistry, and uncomplicated. It is nearly flat lying obscure primary textural-mineralogical re- sheet silicates. with an average southeast dip of 5°. Local lations, textural elements similar to those of folding and brecciation, often accompanied limestone can be identified and their INTRODUCTION by gravity faults, are, however, present. mineralogy defined. Two fundamentally This type of deformation was attributed by different kinds of iron formation are recog- This report deals with the mineralogy Goodwin (1956) to penecontemporaneous nized: (1) cherty iron formation, which and petrography of the relatively un- volcanic disturbances. consists of granules, ooliths, and interstitial metamorphosed Gunflint Iron-Formation The Gunflint Iron-Formation and the cements; and (2) banded or slaty iron for- of Ontario. Emphasis is placed on defining overlying Rove Formation (with which it mation, which is composed of matrices the textural relations and chemistry of forms a gradational contact) comprise the (fine-grained internally structureless silicate- and carbonate-bearing assemb- middle Precambrian Animikie Group. -

Magnetic Properties of Sheet Silicates; 2:1:1 Layer Minerals
Phys Chem Minerals (1985) 12:370-378 PHYSICS CHEMISIRY I]MINERAIS © Springer-Verlag 1985 Magnetic Properties of Sheet Silicates; 2:1:1 Layer Minerals O. Ballet*, J.M.D. Coey and K.J. Burke Department of Pure and Applied Physics, Trinity College, Dublin 2, Ireland Abstract. Magnetization, susceptibility and M6ssbauer tral in chlorites. Substitution of trivalent ions for Mg in spectra are reported for representative chlorite samples with the brucite layers is compensated by replacement of Si by differing iron content. The anisotropy of the susceptibility A1 in the tetrahedral sheets of the talc layers (Bailey 1980). and magnetization of a clinochlore crystal is explained using The general formula for a trioctahedral chlorite is the trigonal effective crystal-field model developed earlier for 1:1 and 2:1 layer silicates, with a splitting of the Tzg [Si4_xAlx]~R2+~3+~c~"1. 6 - x~'~ x J "~ 10krOH~ 18 (1) triplet of 1,120 K. Predominant exchange interactions in where R 2+ is usually Mg or Fe 2+, and R 3+ is usually A1 the iron-rich samples are ferromagnetic with J= 1.2 K, as for other trioctahedral ferrous minerals. A peak in the sus- or Fe 3 +. Typically x --~ 1. The different brackets [ ] and ceptibility of thuringite occurs at Tm = 5.5 K, and magnetic { } are used to denote tetrahedral and octahedral sites re- hyperfine splitting appears at lower temperatures in the spectively. Chlorite minerals rich in ferrous iron are fairly M6ssbauer spectrum. However neutron diffraction reveals common, and a nomenclature has been devised which is no long-range magnetic order in thuringite (or biotite, based on the value of x and the Fe2+/R 2+ ratio (Foster which behaves similarly). -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

Mineralogy and Petrography of Iron Formation at Lake Albanel, Quebec, Canada Mineralogy and Petrography Of
TH 1528 MINERALOGY AND PETROGRAPHY OF IRON FORMATION AT LAKE ALBANEL, QUEBEC, CANADA MINERALOGY AND PETROGRAPHY OF IRON FORMATION AT LAKE ALBANEL Thesis for the Degree of M.S. MICHIGAN STATE UNIVERSITY Rudolph K. Hogberg, 1957 4" i ~1~p n ~/ ({y~~~ 9 f 1t/~ ~8g~~ulpp ® 6 ~~ V> V LL ~~ 11 W WYV ~ ŸT~~ ~ t #âf~'`'. »`{i~Ÿ i~f i,~~l1I1H~ M ~~~ 1Ii~~4~W1 ,~V~ WiY~~~~l ; .~~~~~"Y T6th fol. f t..40 ogir,014 44 m w .5... .1-f: Is.,E1 II ($1x1 ST~~aM UNIVERSIIT',C. ' Rudolph ; 1‹,. ft00.0.rtr 1957 MINERALOGY AND PETROGRAPHY OF IRON FORMATION AT LAKE ALBANEL, QUEBEC, CANADA By RUDOLQH K. HOGBERG A THESIS Submitted to the School of Science and Arts of Michigan State University of Agriculuture and Applied Science. in partial filllmentul of the requirements' for the degree of MASTER OF SCIENCE . Department of Geology 1957 C-, 72-6-7 G . a-44-1 ABSTRACT The Temiscamie River area, on Lake Albanel, is located in the Precambrian shield of westwcentral Quebec, Canada. Results of laboratory examination of forty three thin r•- sections, from diamond drill core fragments, revealed -- certain conclusions relative to textural relationships and mineralogic content of an iron formation. Macroscopic study pointed out three divisions of the Temiscamie iron formation. Two divisions, the "Upper slate" ~ .and the "Lower slate", were used in background study. Micro- __scopic examination of both the "slate" members indicates a striking similarity of alternating light chert and dark extremely fine grained carbonate bands. The middle division, or "Iron formation", consists of anhedral carbonate grains in irregular patches and bean shaped granules composed entirely of carbonate or with varying amounts of chart, all surrounded by an equal-grained chert matrix. -

48. Iron and Carbon Isotope Evidence for Microbial Iron Respiration
Earth and Planetary Science Letters 303 (2011) 121–132 Contents lists available at ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl Iron and carbon isotope evidence for microbial iron respiration throughout the Archean Paul R. Craddock ⁎, Nicolas Dauphas Origins Laboratory, Department of the Geophysical Sciences and Enrico Fermi Institute, The University of Chicago, 5734 South Ellis Avenue, Chicago, IL 60637, United States article info abstract Article history: Banded Iron-Formations (BIFs) are voluminous chemical sediments that are rich in iron-oxide, carbonate and Received 17 August 2010 silica and whose occurrence is unique to the Precambrian. Their preservation in the geological record offers Received in revised form 20 December 2010 insights to the surface chemical and biological cycling of iron and carbon on early Earth. However, many details Accepted 22 December 2010 regarding the role of microbial activity in BIF deposition and diagenesis are unresolved. Laboratory studies have Available online 22 January 2011 + shown that reaction between carbon and iron through microbial iron respiration [2Fe2O3∙nH2O+CH2O+7H → 2+ − Editor: R.W. Carlson 4Fe +HCO3 +(2n+4)H2O+chemical energy] can impart fractionation to the isotopic compositions of these elements. Here, we report iron (δ56Fe, vs. IRMM-014) and carbon isotopic (δ13C, vs. V-PDB) compositions of Keywords: magnetite and of iron-rich and iron-poor carbonates in BIFs from the late Archean (~2.5 Ga) Hamersley Basin, iron-formation Australia and the early Archean (~3.8 Ga) Isua Supracrustal Belt (ISB), Greenland. The range of δ56Fe values Hamersley measured in the Hamersley Basin, including light values in magnetite and heavy values in iron-rich carbonates Isua (up to +1.2‰), are incompatible with their precipitation in equilibrium with seawater. -

Modulated Crystal Structures Of
r63 Thz Canadian M irrcralo g ist vol.36, pp. 163-179(1998) MODULATEDCRYSTAL STRUCTURES OF GREENALITEAND GARYOPILITE: A SYSTEMWITH LONG.RANGE.IN-PLANE STRUCTURAL DISORDER IN THETETRAHEDRA SHEET STEPIIEN GUGGENIIEIM1 Deportmenl of Eanh and Environrnental Sciences, Unhtersity oflllinois at Chicago, 845 W. Taylor Street, Chicago, Illinois 60680, U.S.A. RICHARDA. EGGLETON1 Deparxnent of Geology, Australian National University, Canberra, ACT 0200, Australia Arsrxecr High-resolution transmission electron microscope (TEM) images confirm that greenalite and caryopilite are modulated 1:1 phyllosilicates. The ocrahedrally coordinated Fe (greenalite) and Mn (caryopilite) form trioctahedral sheets.Six-member rings of tetrahedra link to form triangular islands four or five tetrahedra across, with each island coordinatirg 1s sns ssrahedral sheet. Adjacent islands are inverted and link to the neighboring octahedral sheet, which results in a triply-intersecting colTugation for the tetrahedral sheet. Islands vary in numbers of tetrahedra about a mean dictated by the octahedral sheet dimension. Island separationsrange about a mean distance within ttre X-Iplane, with island alignment fluctuating as a function of lattice vectors defined by the octahedral sheet. The tetrahedra ttrus show limited short-range order (spanning to five octahedra), but long-range disorder. Linkages of tetrahedra between islands are apparently completely disordered. Because of this disorder, there is no definable unit-cell. Fourier calculations involving non-repeating structures cannot use unit-cell fractional coordinates and Miller indices. We calculated diffraction patterns by finding the real-space coordinates ^ofevery arcm in the model relative to a defined origin. The reciprocal space variable, d*, is sampled at intervals of0.005 A to build the continuous Fourier transform of the model. -

Minerals of Arizona Report
MINERALS OF ARIZONA by Frederic W. Galbraith and Daniel J. Brennan THE ARIZONA BUREAU OF MINES Price One Dollar Free to Residents of Arizona Bulletin 181 1970 THE UNIVERSITY OF ARIZONA TUCSON TABLE OF CONT'ENTS EIements .___ 1 FOREWORD Sulfides ._______________________ 9 As a service about mineral matters in Arizona, the Arizona Bureau Sulfosalts ._. .___ __ 22 of Mines, University of Arizona, is pleased to reprint the long-standing booklet on MINERALS OF ARIZONA. This basic journal was issued originally in 1941, under the authorship of Dr. Frederic W. Galbraith, as Simple Oxides .. 26 a bulletin of the Arizona Bureau of Mines. It has moved through several editions and, in some later printings, it was authored jointly by Dr. Gal Oxides Containing Uranium, Thorium, Zirconium .. .... 34 braith and Dr. Daniel J. Brennan. It now is being released in its Fourth Edition as Bulletin 181, Arizona Bureau of Mines. Hydroxides .. .. 35 The comprehensive coverage of mineral information contained in the bulletin should serve to give notable and continuing benefits to laymen as well as to professional scientists of Arizona. Multiple Oxides 37 J. D. Forrester, Director Arizona Bureau of Mines Multiple Oxides Containing Columbium, February 2, 1970 Tantaum, Titanium .. .. .. 40 Halides .. .. __ ____ _________ __ __ 41 Carbonates, Nitrates, Borates .. .... .. 45 Sulfates, Chromates, Tellurites .. .. .. __ .._.. __ 57 Phosphates, Arsenates, Vanadates, Antimonates .._ 68 First Edition (Bulletin 149) July 1, 1941 Vanadium Oxysalts ...... .......... 76 Second Edition, Revised (Bulletin 153) April, 1947 Third Edition, Revised 1959; Second Printing 1966 Fourth Edition (Bulletin 181) February, 1970 Tungstates, Molybdates.. _. .. .. .. 79 Silicates ...